© 2025 MJH Life Sciences™ , Patient Care Online – Primary Care News and Clinical Resources. All rights reserved.
9 New Drugs for Primary Care: Q3 2018
Which drugs are most important for your patients? Scroll through our quick slideshow to find out.
It's the end of the third quarter, which means Patient Care has scoured all of the newly FDA-approved drugs and found 9 we believe every primary care physician should know about. Find details in the slideshow below about the first generic version of the EpiPen, a first-in-class oral antibiotic acne treatment, and 7 other noteworthy drugs.

For a quick review of primary care drugs approved in the first quarter of 2018, please go to 9 New Drugs for Primary Care: Q1 2018
For a quick review of primary care drugs approved in the second quarter of 2018, please go to 10 New Drugs for Primary Care: Q2 2018
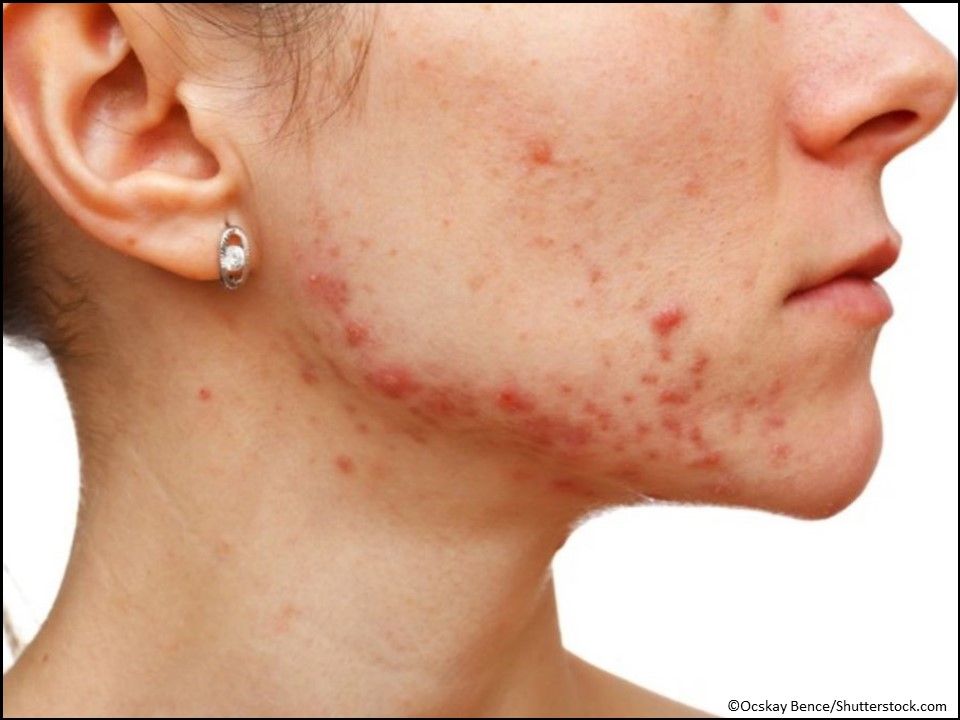
Acne: Seysara (sarecycline) Tablets, a first-in-class tetracycline-derived oral antibiotic for the treatment of inflammatory lesions of non-nodular moderate to severe acne vulgaris for patients aged 9 years or older. (Approved 10/01/18, Almirall, S.A.)
Full prescribing information, click here.
Male Hypogonadism: Xyosted (testosterone enanthate) Subcutaneous Injection, is the first approved subcutaneous testosterone enanthate for once-weekly, self-administration with a single dose auto injector. Xyosted comes in 3 doses (50, 75, and 100 mg) for testosterone replacement therapy in adult males. (Approved 10/01/18, Antares Pharma, Inc.)
Full prescribing information, click here.

Migraine Prophylaxis: Emgality (galcanezumab-gnlm) Injection, is the third calcitonin gene-related peptide (CGRP) antagonist to receive approval for the prevention of migraines in adults. It is used once a month and can be self-administered. (Approved 9/27/18, Eli Lilly and Company)
Full prescribing information, click here.
Migraine Prophylaxis: Ajovy (fremanezumab-vfrm) Injection, is the second CGRP inhibitor to get approved for the prevention of migraines in adults. Ajovy is the first and only anti-CGRP treatment to have quarterly (675 mg) and monthly (225 mg) dosing options, Teva Pharmaceuticals announced in a press release. (Approved 9/14/18, Teva Pharmaceuticals USA, Inc.)
Full prescribing information, click here.
Opiate Dependence: Cassipa (buprenorphine and naloxone) Sublingual Film, is a partial-opioid agonist (buprenorphine 16mg) and opioid antagonist (naloxone 4mg) combination that is applied under the tongue for the maintenance treatment of opioid dependence. (Approved 9/07/18, Teva Pharmaceuticals USA, Inc.)
Full prescribing information, click here.
HIV Infection: Pifeltro (doravirine) Tablets, non-nucleoside reverse transcriptase inhibitors to be administered along with other antiretroviral medicines for the treatment of HIV-1 infection in adults with no prior antiretroviral treatment. (Approved 8/30/18, Merck)
Full prescribing information, click here.
HIV Infection: Delstrigo (doravirine, lamivudine and tenofovir disoproxil fumarate) Tablets, a once-daily fixed dose combination of doravirine (100 mg), lamivudine (300 mg), and tenofovir disoproxil fumarate (300 mg) for the treatment of HIV-1 infection in adults with no prior antiretroviral treatment. (Approved 8/30/18, Merck)
Full prescribing information, click here.
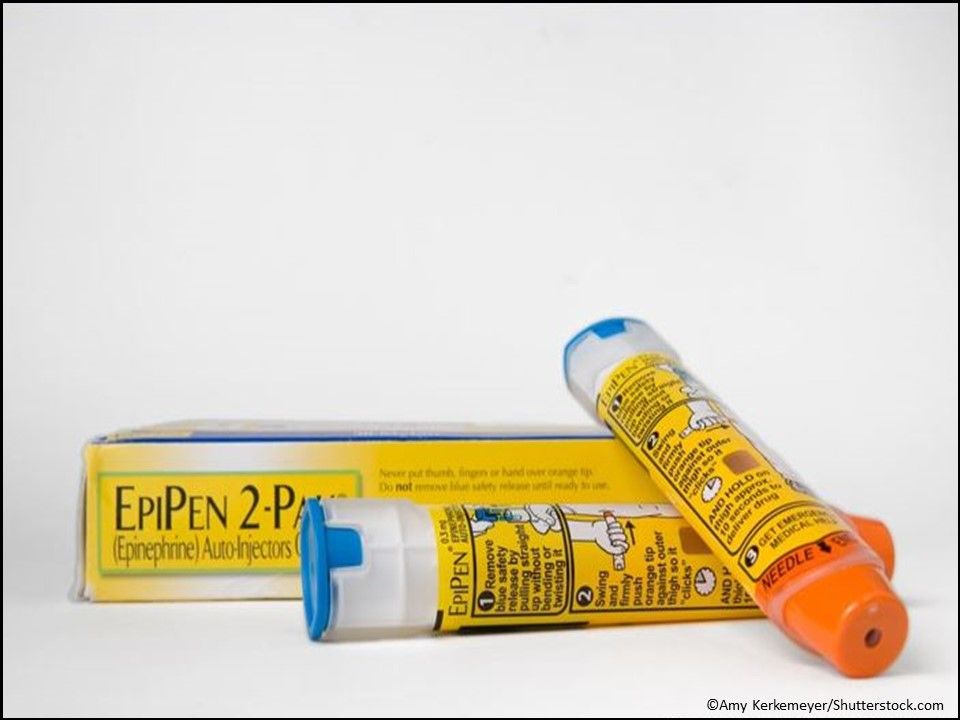
Allergic Reactions: Epinephrine (Autoinjector) Injection (EpiPen generic), is the first generic version of EpiPen and EpiPen Jr to receive approval for the emergency treatment of allergic reactions in adults and pediatric patients who weigh more than 33 lbs. The epinephrine autoinjector comes in 0.3 mg and 0.15 mg doses. (Approved 8/16/18, Teva Pharmaceuticals USA, Inc.)
Full prescribing information, click here.
Pulmonary Arterial Hypertension: Implantable System for Remodulin (treprostinil) (ISR), is a new drug application for the use of Remodulin injection. Remodulin received approval to treat PAH by continuous subcutaneous and intravenous routes of administration back in 2002 and 2004, respectively, however the ISR will provide patients a new delivery option. The ISR is implanted into the body and refilled by a healthcare professional at intervals of up to 16 weeks depending on the dose. (Approved 7/31/18, United Therapeutics Corp) (FDA recently approved medical devices)
