© 2025 MJH Life Sciences™ , Patient Care Online – Primary Care News and Clinical Resources. All rights reserved.
10 New Drugs for Primary Care: Q2 2018
What new drugs should you know about from the second quarter of 2018? Scroll through our quick slideshow to find out.

10 New Drugs for Primary Care: Second Quarter, 2018. At the close of the second quarter, Patient Care has compiled a visual list of 10 new FDA-approved drugs that every primary care physician should know about. Find details in our slide show about the first cannabis-based drug sanctioned by FDA; an oral drug that helps treat both hypertension and osteoarthritis; the first CGRP inhibitor approved for migraine prevention, plus 7 more.
For a quick review of primary care drugs approved in the first quarter of 2018, please go to 9 New Drugs for Primary Care: Q1 2018

Epilepsy: Epidiolex (cannabidiol) Oral Solution, the first cannabis-based drug to receive FDA approval for the treatment of two rare and severe forms of epilepsy, Lennox-Gastaut syndrome and Dravet syndrome, in patients aged 2 years and older. (Approved 6/25/18, GW Pharmaceuticals plc)
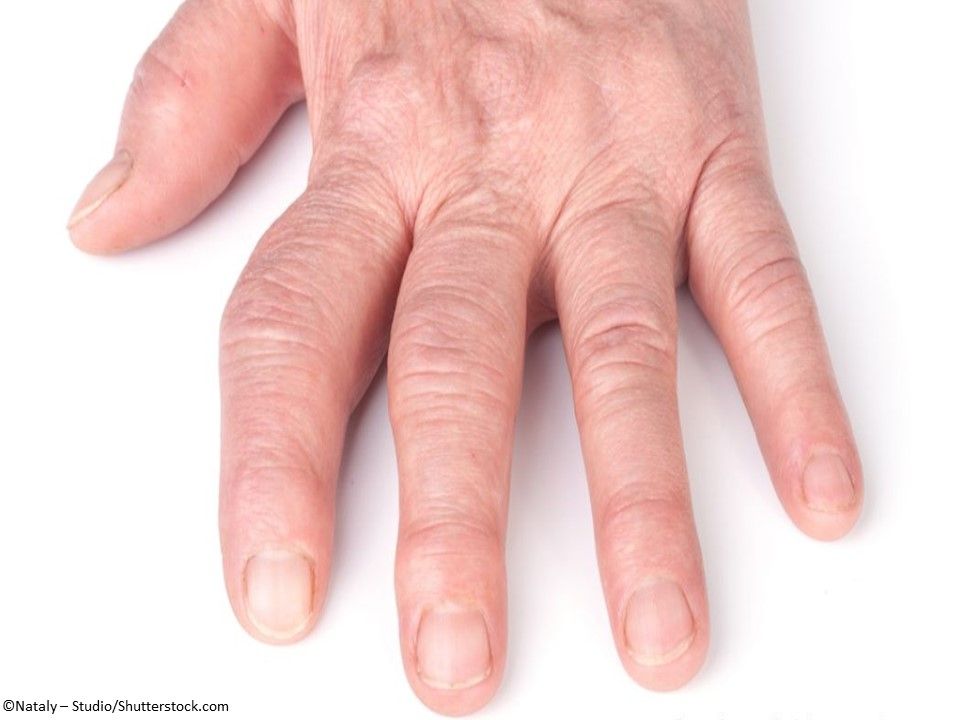
Rheumatoid Arthritis: Olumiant (baricitinib) Tablets, a 2-mg, once-daily oral Janus kinase inhibitor for the treatment of adults with moderate-to-severe active RA who have had an inadequate response to 1 or more tumor necrosis factor inhibitor therapies. (Approved 6/1/2018, Eli Lilly and Company)

Hypertension/Osteoarthritis: Consensi (amlodipine and celecoxib) Tablets (formerly KIT-302), a combination of an antihypertensive calcium channel blocker (amlodipine besylate) and a nonsteroidal anti-inflammatory drug (celecoxib) approved for once daily use in three dosage forms. The dosage corresponds to the current approved dosages of amlodipine (2.5, 5, and 10 mg) for hypertension and a 200 mg dose of celecoxib for the treatment of osteoarthritis pain. (Approved 5/31/18, Kitov Pharma Ltd)

Tadalafil Tablets (Cialis Generic), a phosphodiesterase-5 enzyme inhibitor to treat erectile dysfunction, symptoms of benign prostatic hypertrophy, and pulmonary arterial hypertension. Its dosage ranges from 2.5 mg to 20 mg. (Approved 5/22/18, Teva Pharmaceuticals USA, Inc.)
Hyperkalemia: Lokelma (sodium zirconium cyclosilicate) for Oral Suspension (formerly ZS-9), an insoluble, non-absorbed sodium zirconium silicate that acts as a highly selective potassium-removing agent. It is administered orally as an odorless and tasteless powder reconstituted in water. (Approved 5/18/18, AstraZeneca)

Migraine Prophylaxis: Aimovig (erenumab-aooe) Injection, the first of 4 calcitonin gene related peptide (CGRP) inhibitors to receive approval for the prevention of migraines in adults that works by targeting and blocking the CGRP receptor. (Approved 5/17/18, Amgen Inc.)

Opiate Withdrawal: Lucemyra (lofexidine hydrochloride) Tablets, a selective alpha 2-adrenergic receptor agonist that reduces the release of norepinephrine to decrease the severity of withdrawal symptoms in patients experiencing opioid withdrawal. Lucemyra does not completely prevent withdrawal symptoms and is only approved for treatment for up to 14 days. (Approved 5/16/18, US WorldMeds)
Bowel Preparation: Plenvu (polyethylene glycol 3350 with electrolytes) for Oral Solution, a lower-volume, polyethylene glycol based osmotic laxative indicated for cleansing of the colon before a colonoscopy. (Approved 5/4/18, Salix Pharmaceuticals, Inc.)
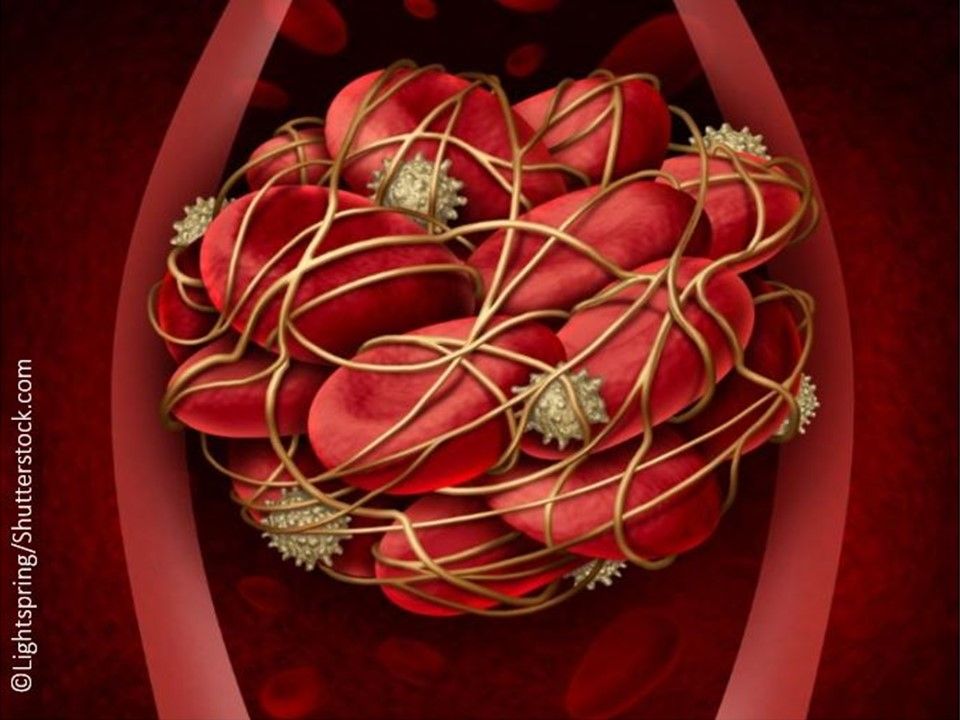
Reversal of Anticoagulant Activity of Factor Xa Inhibitors: Andexxa (coagulation factor Xa (recombinant), inactivated-zhzo), a recombinant modified human factor Xa protein indicated for patients treated with rivaroxaban and apixaban, when reversal of anticoagulation is needed due to life-threatening or uncontrolled bleeding. (Approved 5/3/18, Portola Pharmaceuticals, Inc.)
HIV: Symfi (efavirenz, lamivudine and tenofovir disoproxil fumarate) Tablets, a three-drug combination of a non-nucleoside reverse transcriptase inhibitor (600mg of efavirenz) and 2 nucleo(t)side reverse transcriptase inhibitors (300mg of lamivudine and 300mg of tenofovir disoproxil fumarate) indicated as a complete regimen for the treatment of human immunodeficiency virus type 1 (HIV-1) infection in adult and pediatric patients weighing at least 40 kg. (Approved 4/22/18, Mylan Pharmaceuticals Inc.)
