© 2025 MJH Life Sciences™ , Patient Care Online – Primary Care News and Clinical Resources. All rights reserved.
8 Questions on Personalized Medicine for Cystic Fibrosis
Take this brief test to find out what you know about key developments in the evolution of this emerging treatment option.
A promising approach to cystic fibrosis (CF) treatment has been found in personalized medicine, also known as precision medicine, in which interventions are personalized to individual patients. Take this brief, 8-question quiz to find out what you know about key developments in the evolution of this emerging treatment option.

Question 1. The precision medicine approach to disease treatment and prevention takes into account patients’ individual variability in which factors?

Answer: C. Genes, environment, and lifestyle. With consideration of these factors, the precision medicine approach may be used to more accurately predict which treatment and prevention strategies will work in which groups of patients rather than one in which strategies are developed with less consideration for differences between patients.
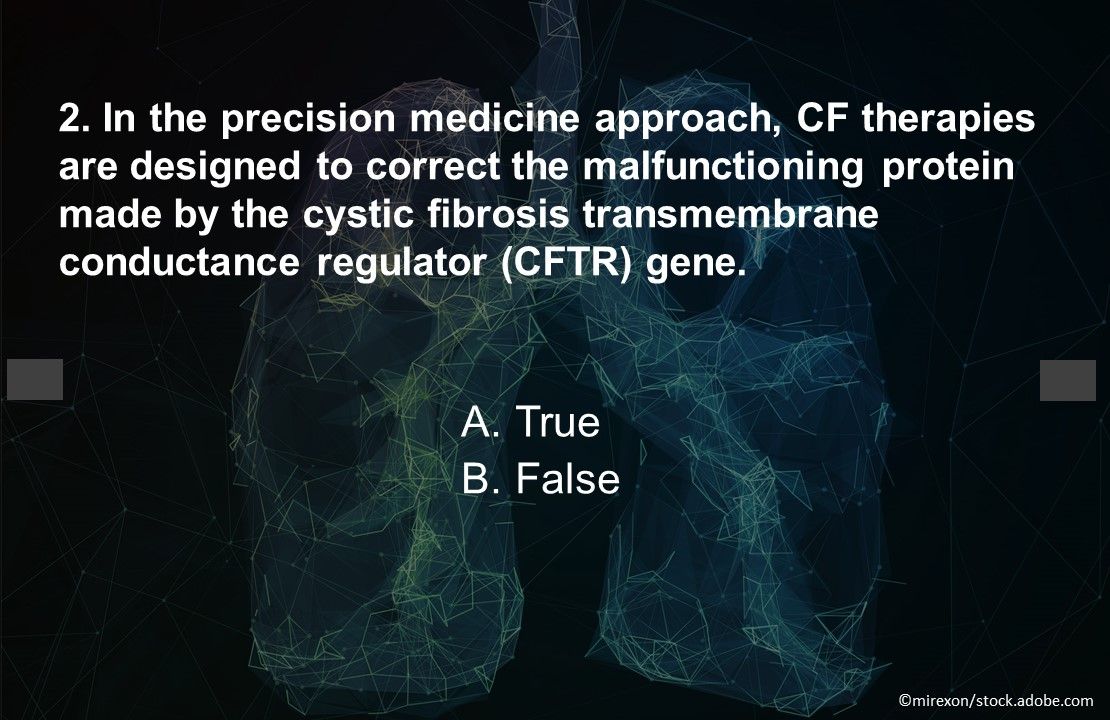
Question 2. True or false? In the precision medicine approach, CF therapies are designed to correct the malfunctioning protein made by the CFTR gene.

Answer: A. True. Existing CFTR modulator therapies include ivacaftor (Kalydeco), lumacaftor/ivacaftor (Orkambi), tezacaftor/ivacaftor (Symdeko), and elexacaftor/tezacaftor/ivacaftor (Trikafta). More are in development.
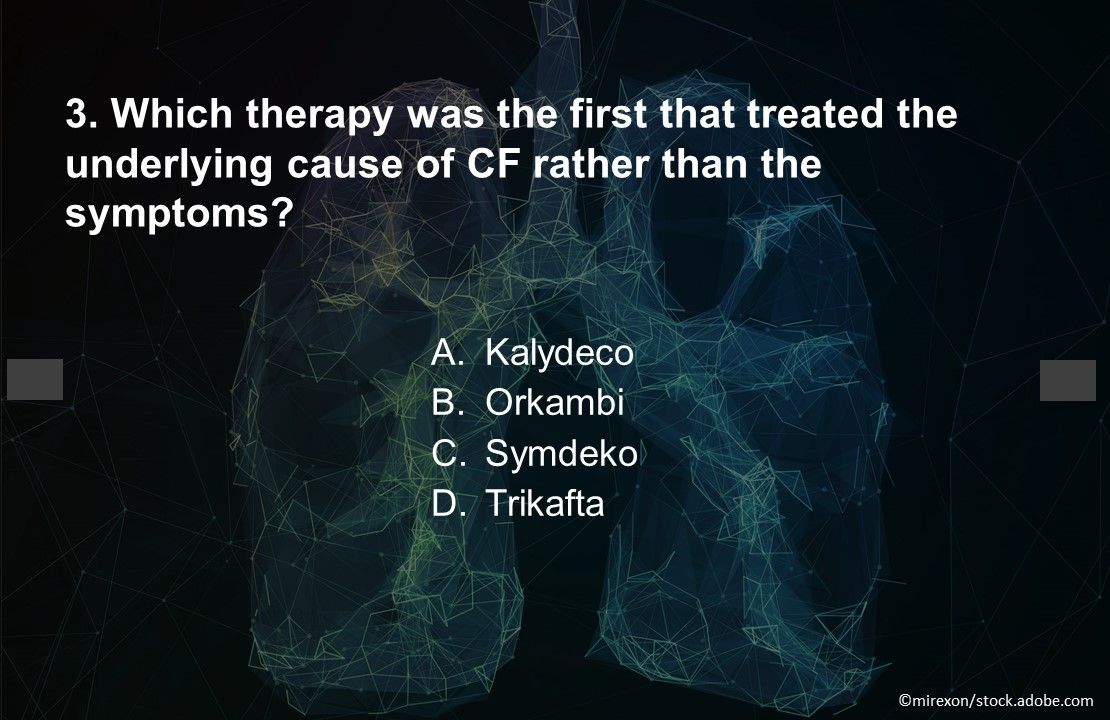
Question 3. Which therapy was the first that treated the underlying cause of CF rather than the symptoms?
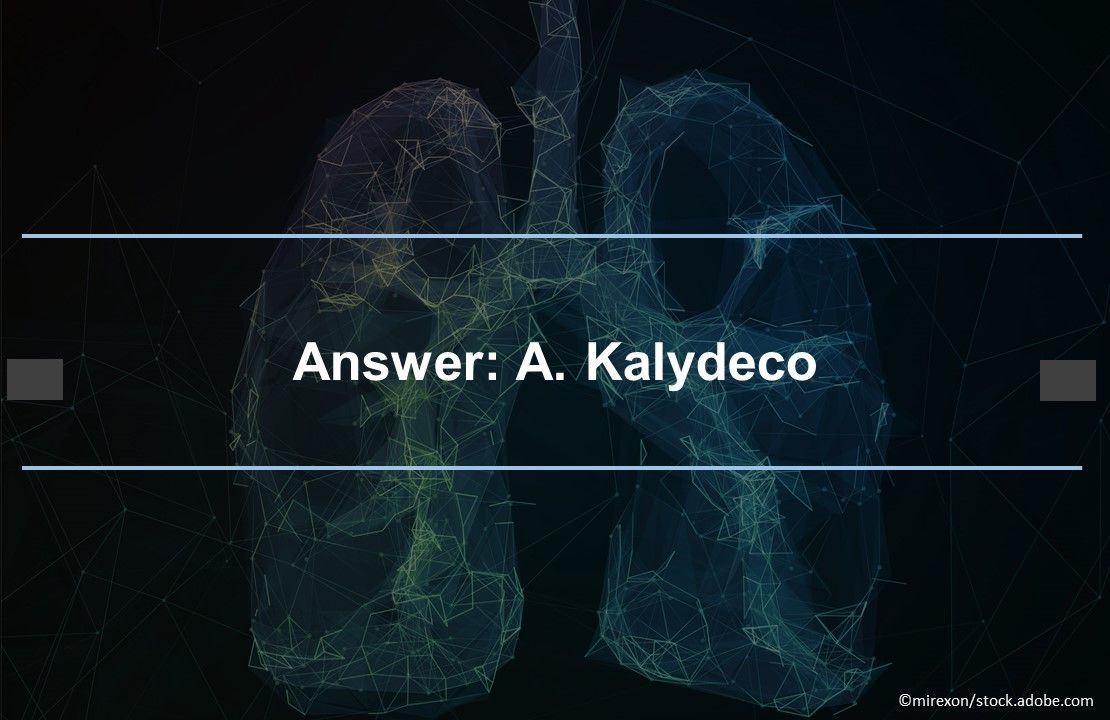
Answer: A. Kalydeco. Kalydeco was FDA-approved in 2012 to treat the G551D CFTR gene variant that causes 3%-4% of CF cases. The FDA expanded the label to 9 additional mutations in 2014 and to 28 more in 2018, tripling eligibility to 14% of the CF population. Ivacaftor significantly decreased pulmonary morbidity, lowered hospitalization rates, and improved weight gain and quality of life.
Answer: B. F508del. For treating the underlying cause of CF in patients aged ≥2 years with 2 copies of the F508del mutation in their CFTR gene (about half of CF cases), the FDA first approved Orkambi (a CFTR potentiator and CFTR corrector combination) in 2015.
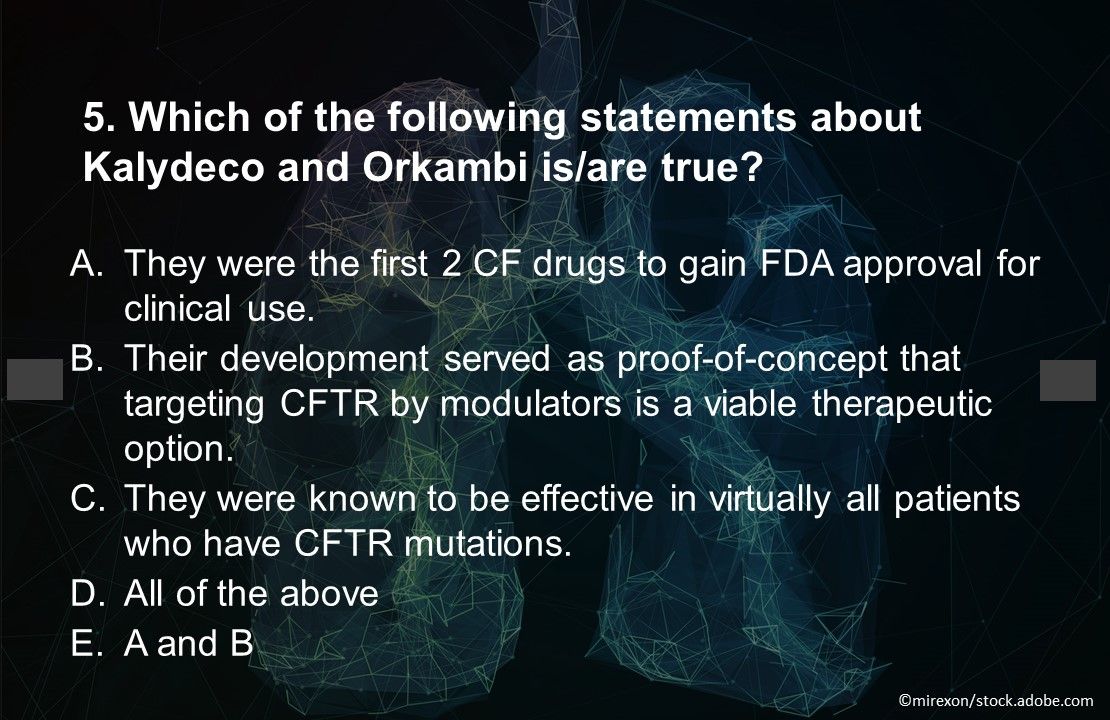
Answer: E. A and B. The authors of a 2018 review stated that although the agents’ development and approval served as proof-of-concept that targeting CFTR by modulators is a viable therapeutic option, they were known to be effective only in patients who had certain CFTR mutations, leaving many others without similar treatment options.
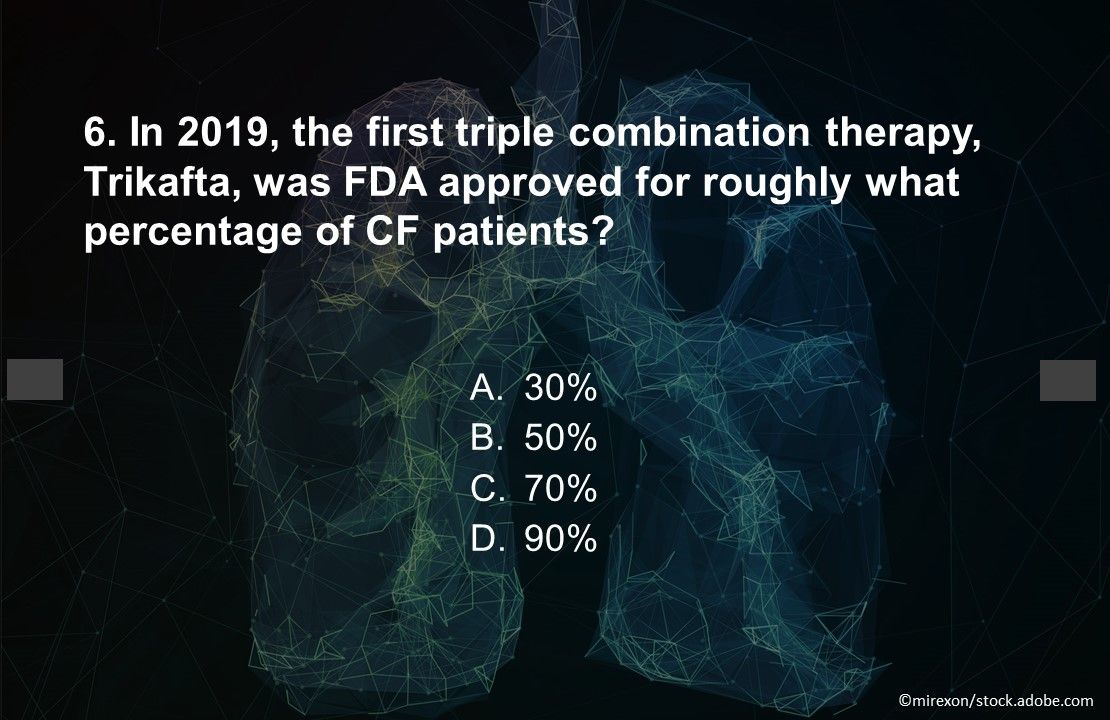
Question 6. In 2019, the first triple combination therapy, Trikafta, was FDA approved for roughly what percentage of CF patients?
Answer: D. 90%. Trikafta was approved for patients aged ≥12 years who have at least 1 F508del mutation in the CFTR gene. Study results in the New England Journal of Medicine showed the combination to be efficacious in patients with Phe508del–minimal function genotypes in whom previous CFTR modulator regimens were ineffective.
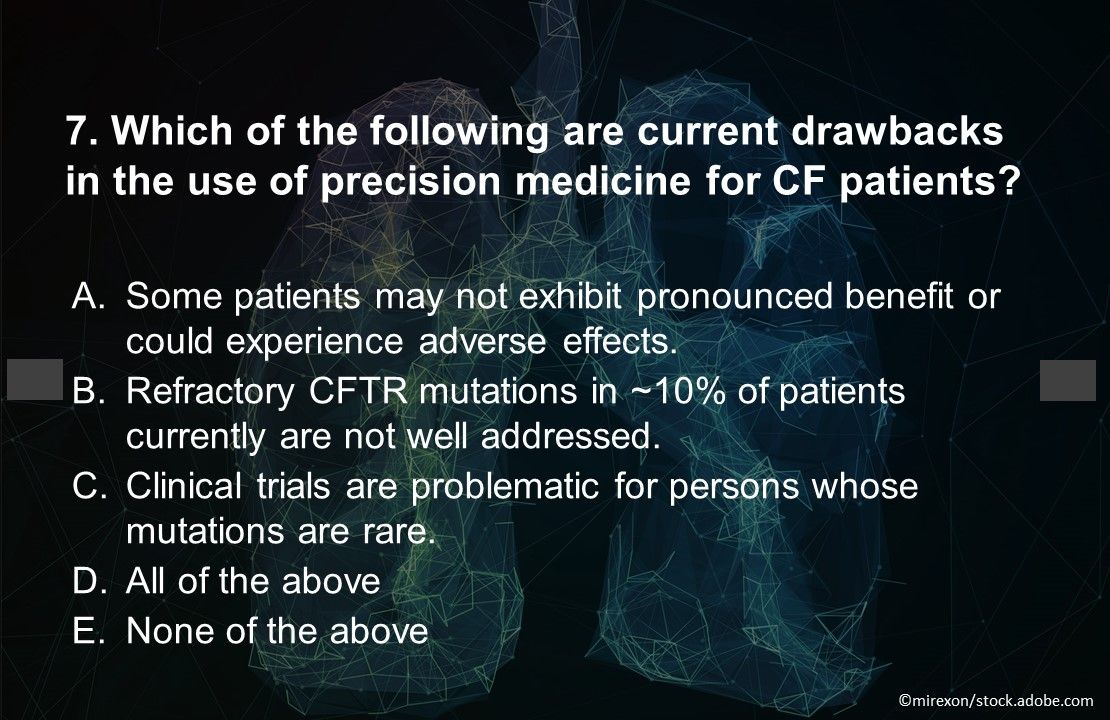
Question 7. Which of the above are current drawbacks in the use of precision medicine for CF patients?
Answer: D. All of the above. In spite of the challenges, triple combination therapies and other CFTR modulators promise to offer benefit for most patients with CF.
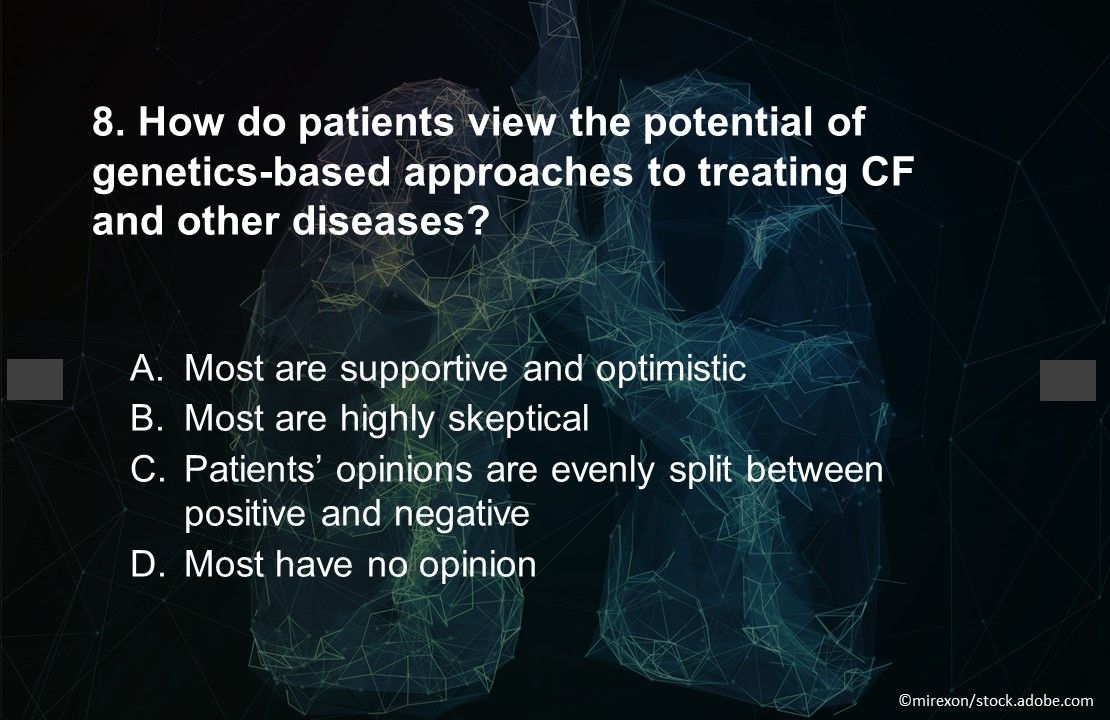
Question 8. How do patients view the potential of genetics-based approaches to treating CF and other diseases?
Answer: A. Most are supportive and optimistic. Among respondents to a 2020 survey, 78% said they thought researchers will use genetics to find cures for cancer, Alzheimer, and other diseases; 71% said physicians will be able to use genetic information to inform their health care; and 60% thought changing genes in embryos could prevent CF, sickle cell disease, and other severe diseases.
For more information on all questions and answers, please visit Cystic Fibrosis and Personalized Medicine: A Brief History.