© 2025 MJH Life Sciences™ , Patient Care Online – Primary Care News and Clinical Resources. All rights reserved.
The Obesity Drug Pipeline: 10 Questions on Rx Development
Take this quick quiz to test your knowledge of recent advances most likely to have an impact on weight management in your practice.
Treatments for patients with obesity are in development across the pharmaceutical industry, including molecules with novel mechanisms of action that have been evaluated in clinical trials but have not yet received FDA approval.
Take this quick quiz to test your knowledge of the recent advances most likely to have an impact on weight management in your practice.
2. In a recent trial of once-weekly subcutaneous semaglutide 2.4 mg plus lifestyle intervention, what percentage of adults with overweight or obesity attained at least 5% weight loss?
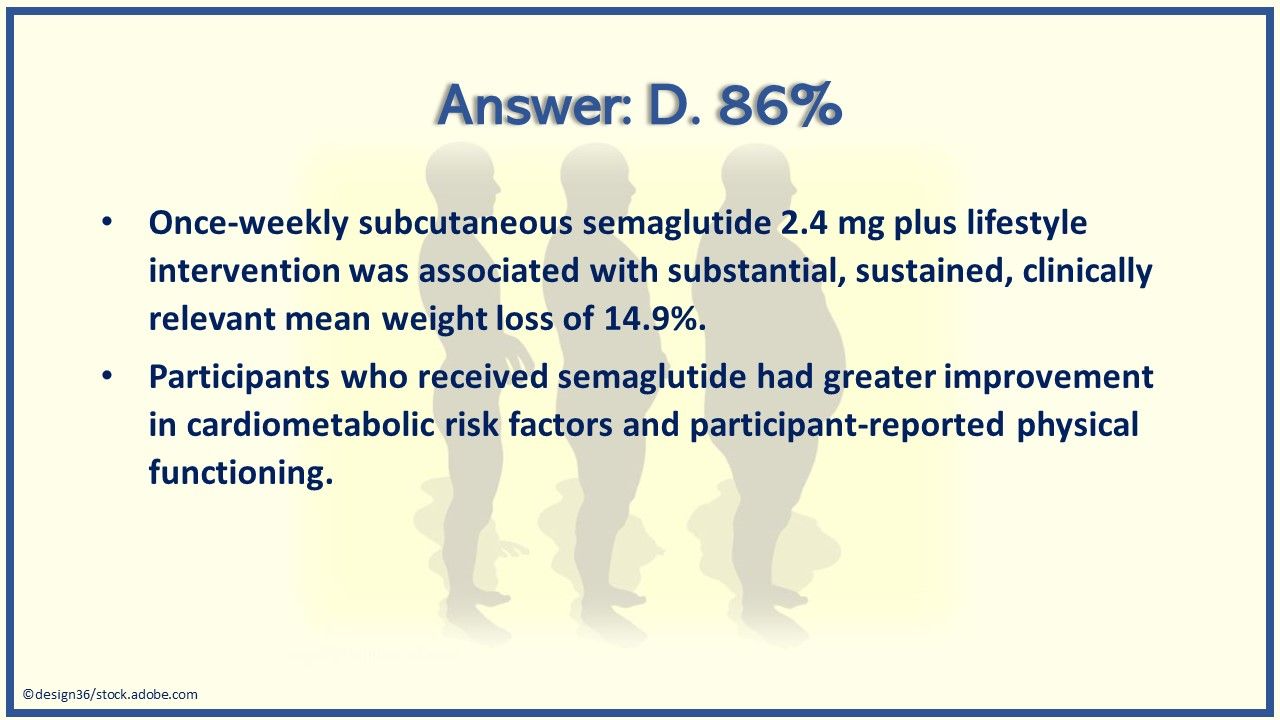
Answer: D. 86% of patients with overweight lost at least 5% of their weight in a recent trial of once-weekly semaglutide 2.4 mg.
3. Which medication gained FDA approval for chronic weight management in adults with general obesity or overweight in June 2021?

Answer: A. Semaglutide gained FDA approval for chronic weight management in adults with general obesity or overweight in June 2021.

4. In a recent weight management trial, what result was achieved with cagrilintide plus semaglutide compared with semaglutide alone?

Answer: C. Participants who took cagrilintide plus semaglutide achieved significantly greater weight loss compared with those who took semaglutide alone.

5. Once-weekly cagrilintide led to significant reductions in body weight and was well tolerated in a phase 2 trial. True or False?

A. True. Mean percentage weight reductions were greater with all doses of cagrilintide vs placebo, and weight reductions were greater with cagrilintide 4.5 mg vs liraglutide 3.0 mg.

6. Which medication under development for type 2 diabetes (T2D) reduced weight in participants in the phase 3 SURPASS-2 trial?

7. In a phase 2 trial, blockade of activin type II receptors (ActRII) with bimagrumab had what result for patients with overweight or obesity who had T2D?

Answer: D. All the above. Use of bimagrumab led to significant loss of total body fat mass, gain in lean mass, and metabolic improvements.
8. Which medication received FDA approval for chronic weight management in patients with obesity caused by rare genetic disorders?

Answer: B. Setmelanotide. In November 2020, setmelanotide received approval for patients aged ≥6 years with obesity caused by pro-opiomelanocortin, proprotein convertase subtilisin/kexin type 1, or leptin receptor deficiency.

9. Oral superabsorbent hydrogel (Plenity) was FDA approved as a medicine for treatment of overweight and obesity in 2019. True or False?

Answer: B. False. Plenity did receive FDA approval for overweight and obesity in 2019, but as a medical device, not a medicine.

10. Which of the obesity drug therapies currently in the pipeline is a first-in-class leptin sensitizer?

Answer: C. ERX1000 is a first-in-class leptin sensitizer in development by ERX Pharmaceuticals.
Please see "Obesity Drugs in Development: A brief overview of the pipeline: for additional information. https://www.patientcareonline.com/view/obesity-drugs-in-the-pipeline-a-brief-overview-of-the-pipeline







