© 2025 MJH Life Sciences™ , Patient Care Online – Primary Care News and Clinical Resources. All rights reserved.
PCV13: Effective Against First-Episode CAP in Older Adults
The 13-valent pneumococcal conjugate vaccine reduced nearly by half vaccine-type community acquired pneumonia in adults aged 65 years and older.

Bonten MJM, Huihts SM, Bolkenbaas M, et al. Polysaccharide conjugate vaccine against pneumococcal pneumonia in adults. N Engl J Med. 2015;372:1114-1125.

Randomized, double-blind, placebo-controlled CAPITA trial* used a serotype-specific urinary antigen detection assay to test whether a 13-valent pneumococcal conjugate vaccine (PCV13) is effective for preventing vaccine-type invasive and noninvasive community-acquired pneumonia in adults aged 65 and older.More information, here.

84,496 adults aged 65 years and older were randomly assigned to placebo or PCV13 and evaluated for prevention of first episodes of: Vaccine-type strains of pneumococcal, community-acquired pneumonia, nonbacteremic and noninvasive pneumococcal community-acquired pneumonia, invasive pneumococcal disease.
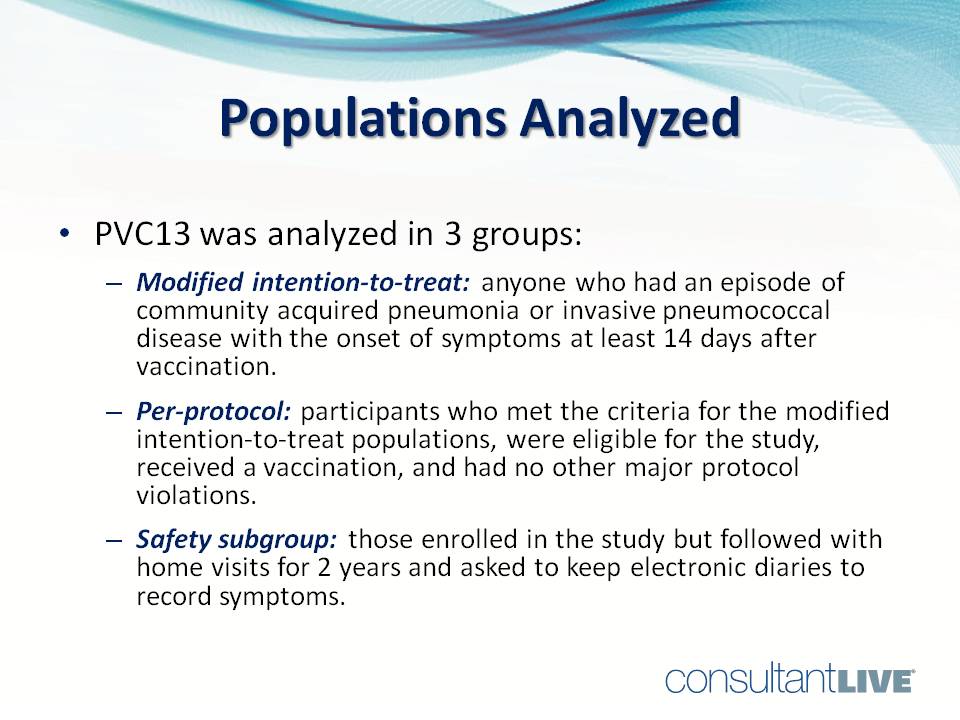
PVC13 was analyzed in 3 groups: Modified intention-to-treat: anyone who had an episode of community-acquired pneumonia or invasive pneumococcal disease with the onset of symptoms at least 14 days after vaccination. Per-protocol: participants who met the criteria for the modified intention-to-treat populations, were eligible for the study, received a vaccination, and had no other major protocol violations. Safety subgroup: those enrolled in the study but followed with home visits for 2 years and asked to keep electronic diaries to record symptoms.
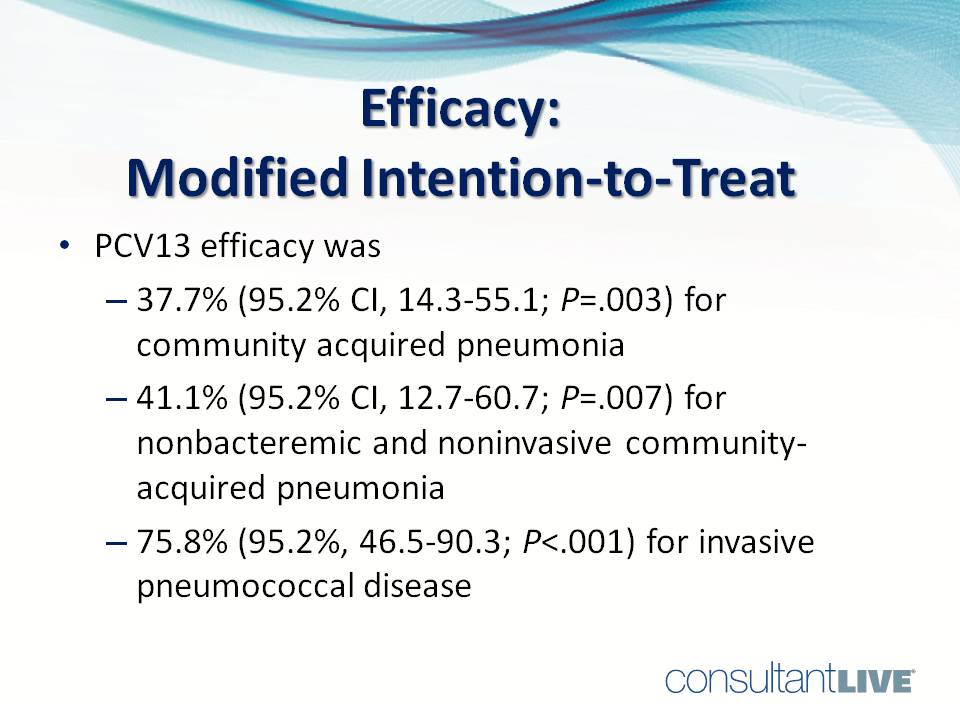
PCV13 efficacy was: 37.7% (95.2% CI, 14.3-55.1; P=.003) for community-acquired pneumonia; 41.1% (95.2% CI, 12.7-60.7; P=.007) for nonbacteremic and noninvasive community-acquired pneumonia; 75.8% (95.2% CI, 46.5-90.3; P

Local reactions and systemic events were more frequent in the vaccine group, but overall were mild or moderate. More patients given PCV13 had an adverse event within 1 month of vaccination (18.7 vs 14.3; P=.01). No difference in newly diagnosed chronic medical conditions, serious adverse events, or deaths were found between the two study groups.
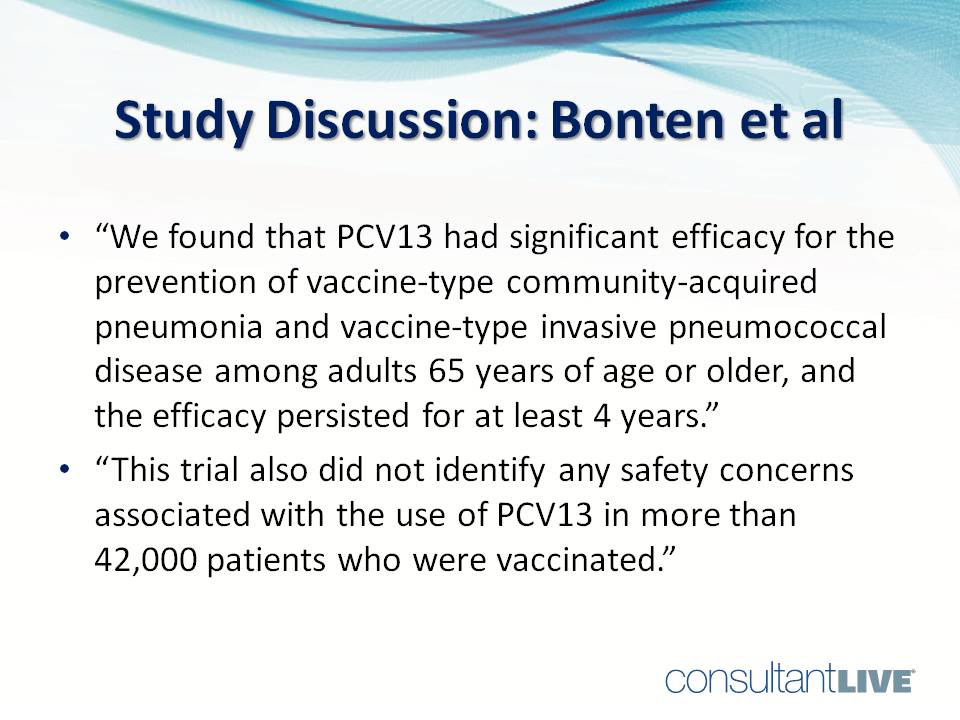
“We found that PCV13 had significant efficacy for the prevention of vaccine-type community-acquired pneumonia and vaccine-type invasive pneumococcal disease among adults 65 years and older, and the efficacy persisted for at least 4 years. . . . This trial also did not identify any safety concerns associated with the use of PCV13 in more than 42,000 patients who were vaccinated.”
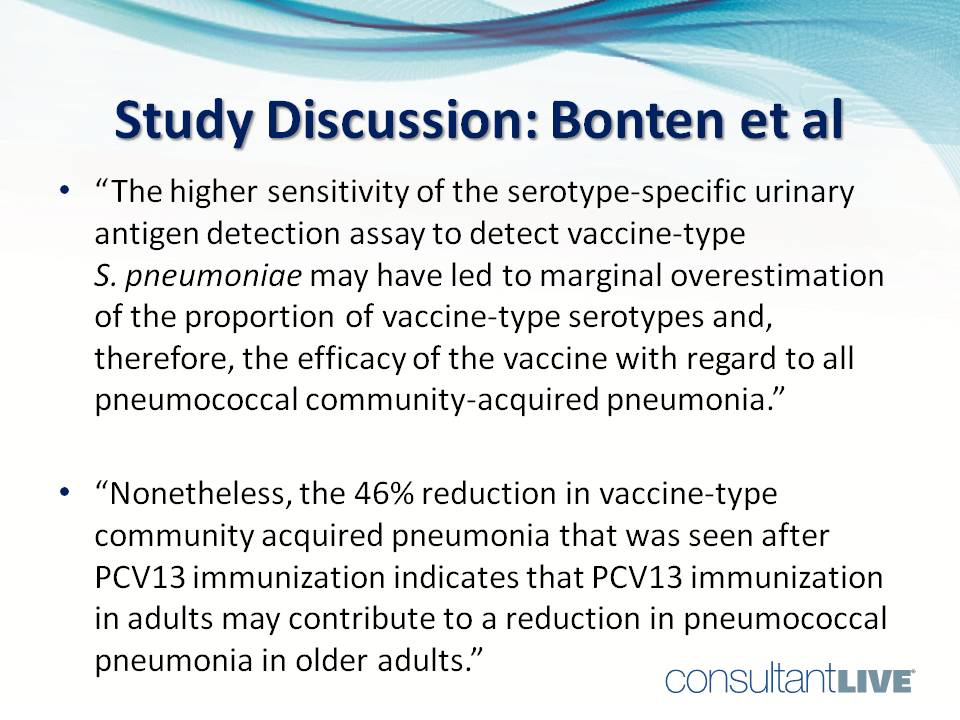
“The higher sensitivity of the serotype-specific urinary antigen detection assay to detect vaccine-type S pneumoniae may have led to marginal overestimation of the proportion of vaccine-type serotypes and, therefore, the efficacy of the vaccine with regard to all pneumococcal community-acquired pneumonia. . . . Nonetheless, the 46% reduction in vaccine-type community-acquired pneumonia that was seen after PCV13 immunization indicates that PCV13 immunization in adults may contribute to a reduction in pneumococcal pneumonia in older adults.”




