© 2025 MJH Life Sciences™ , Patient Care Online – Primary Care News and Clinical Resources. All rights reserved.
Gabapentinoids used Off-label for Pain: Effective or Erroneous?
Off-label gabapentin and pregabalin prescribing for pain is widespread; the opioid crisis may be to blame. But does science support the trend? Find out with these 10 questions.
The heightened focus and widening regulatory restrictions on opioid prescribing may be prompting more widespread use of alternatives to this class of medications. Two drugs that are increasingly being prescribed for pain management are the gabapentinoids gabapentin (Neurontin) and pregabalin (Lyrica). There are many questions, however, about their efficacy for the numerous pain conditions for which they are prescribed, off-label. (Contiued below)

A recent review in JAMA Internal Medicine of the evidence for off-label use of gabapentinoids for pain management is highly instructive. The 10 questions in the slides above are based on the results. See what you know about the drugs, their popularity, and the facts.
Answer: B. Postherpetic neuralgia
Postherpetic neuralgia is the only pain condition for which gabapentin is approved by the FDA.
Answer: F. All of the above
Pregabalin is FDA-approved for the treatment of fibromyalgia, postherpetic neuralgia, diabetic neuropathic pain, and pain associated with spinal cord injury.
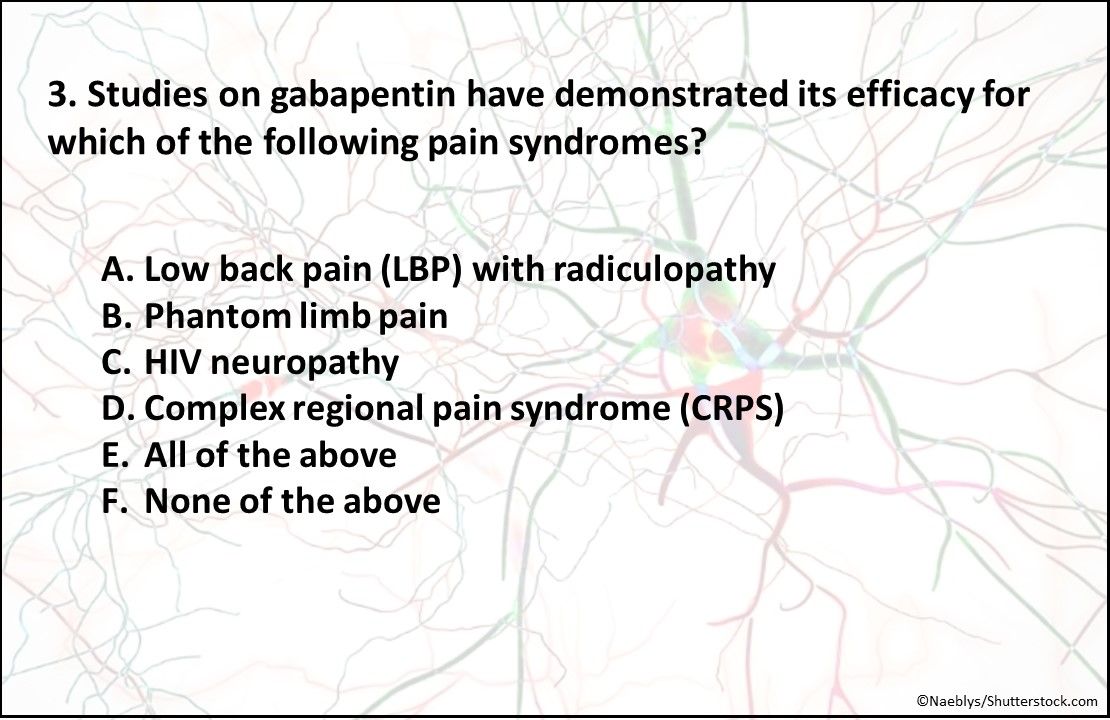
3. Studies on gabapentin have demonstrated its efficacy for which of the pain syndromes listed above?

Answer: F. None of the above
Studies have been performed on the use of gabapentin for LBP with radiculopathy, phantom limb pain, HIV neuropathy, and CRPS, but have found little or no difference between its benefits and those from placebo.
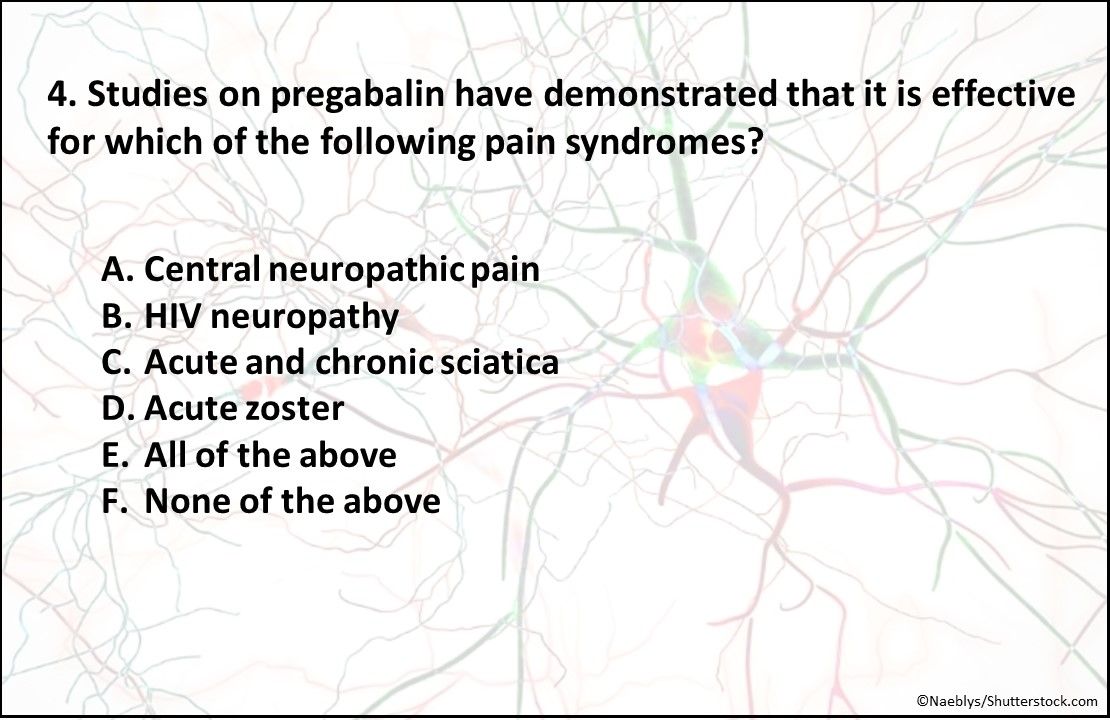
4. Studies on pregabalin have demonstrated that it is effective for which of the pain syndromes listed above?

Answer: F. None of the above
Pregabalin has been studied for the treatment of central neuropathic pain; HIV neuropathy; acute and chronic sciatica; and acute zoster. However, the studies found that it was no more effective vs placebo.
5. Between 2002 and 2015, how did the percentage of individuals who used gabapentin and/or pregabalin change?
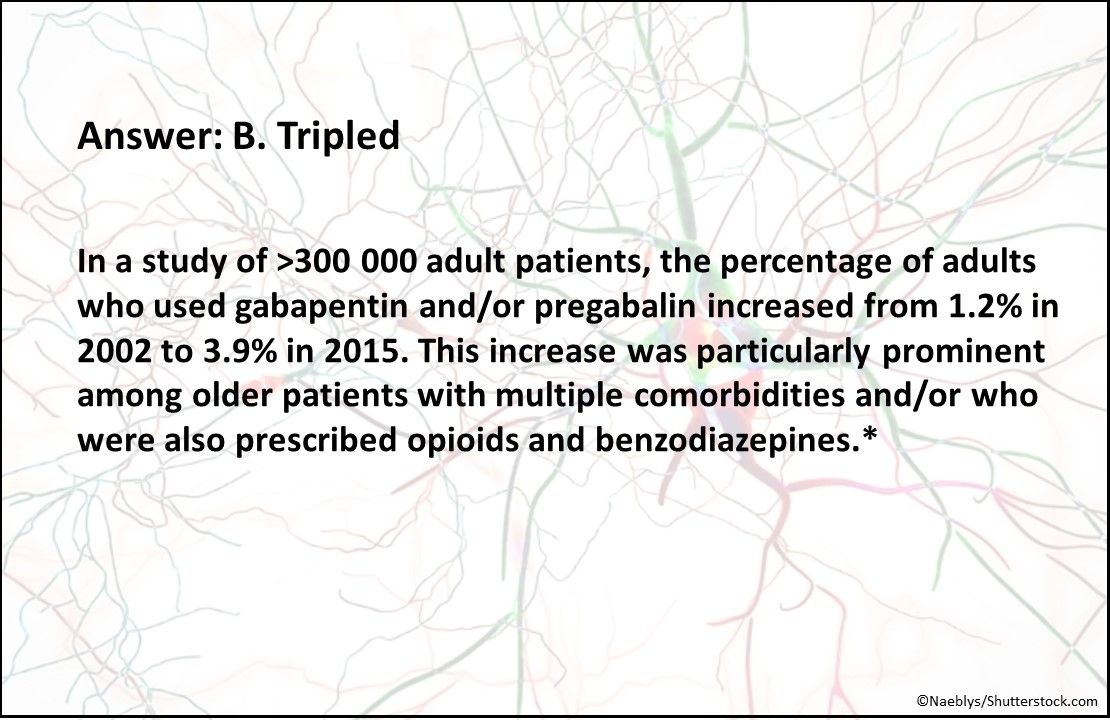
Answer: B. Tripled
In a study* of >300 000 adult patients, the percentage who used gabapentin and/or pregabalin increased from 1.2% in 2002 to 3.9% in 2015. This increase was particularly prominent among older patients with multiple comorbidities and in those also prescribed opioids and benzodiazepines.*
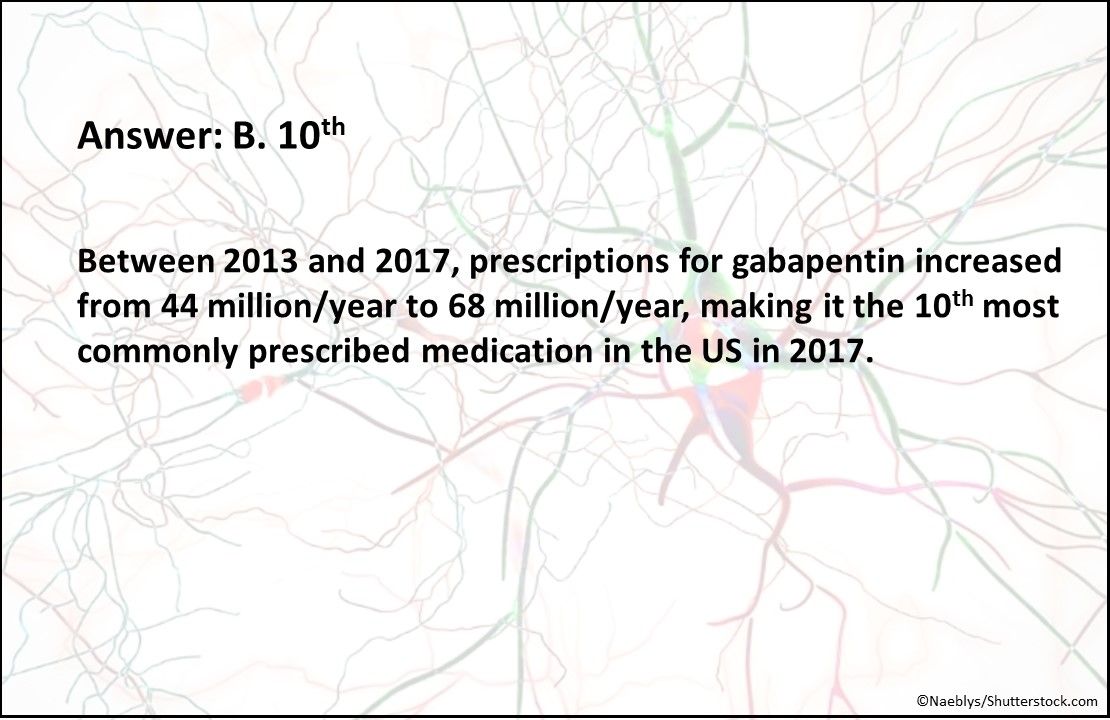
Answer: B. 10th
Between 2013 and 2017, prescriptions for gabapentin increased from 44 million/year to 68 million/year, making it the 10th most commonly prescribed medication in the US in 2017.
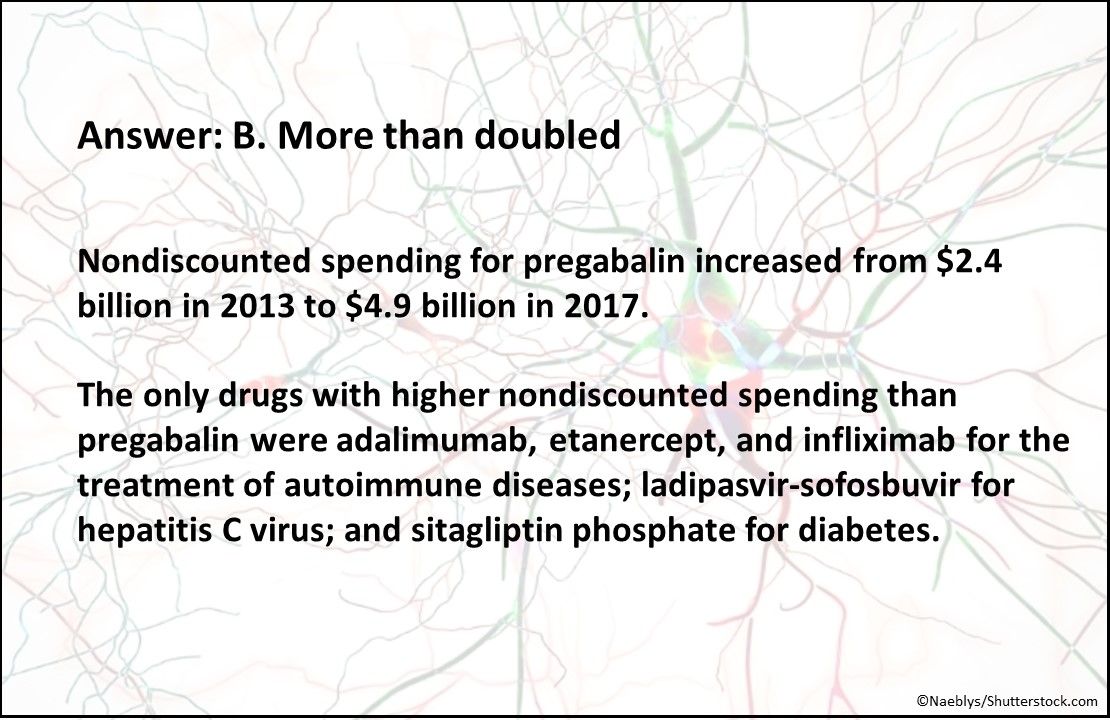
Answer: B. More than doubled
Nondiscounted spending for pregabalin increased from $2.4 billion in 2013 to $4.9 billion in 2017. The only drugs with higher nondiscounted spending than pregabalin were adalimumab, etanercept, and infliximab for the treatment of autoimmune diseases; ladipasvir-sofosbuvir for hepatitis C virus; and sitagliptin for diabetes.

8. True or False. Pregabalin was FDA-approved for the management of fibromyalgia (vs gabapentin) because pregabalin has been shown to be far more effective than gabapentin for the condition.
Answer: B. False
Although pregabalin is FDA-approved for the management of fibromyalgia, gabapentin is often prescribed off-label, likely due to its lower generic pricing (pregabalin is marketed exclusively as Lyrica). Studies have found them similarly effective for fibromyalgia, although the efficacy is limited for both.
9. What is the level of the evidence to support the now common off-label prescribing of gabapentin for diabetic neuropathic pain?

Answer: B. Mixed
Among the 5 double-blind, placebo-controlled studies on gabapentin for diabetic neuropathic pain, 2 showed no benefit vs placebo and 2 found it only minimally more effective vs placebo (mean difference in pain ~1 on a 0-10 scale).

Answer: E. A, B, and C
Somnolence, dizziness, and gait disturbances are consistently found to be the most common adverse effects associated with the use of gabapentinoids. Whether the difference in adverse effects between pregabalin and gabapentin is meaningful is currently unclear.
Dr King's Take Home Points
- Rising use of gabapentinoids for pain is not supported by new FDA-approved indications or an increase prevalence of treated pain conditions.
- It is likely the accelerted prescribing is in part related to gabapentinoids serving as an alternative to opioids.
- There is limited evidence that gabapentinoids are effective for the range of pain conditions for which they are prescribed.
- The perception that gabapentinoids, because of their approved indication to treat pain with neuropathic components, will be effective for any pain presumed to be "neuropathic" is erroneous.
Source: Goodman CW, Brett AS. A clinical overview of off-label use of gabapentinoid drugs. JAMA Intern Med. 2019;179:695-701.





