© 2025 MJH Life Sciences™ , Patient Care Online – Primary Care News and Clinical Resources. All rights reserved.
Are Biologics for COPD Around the Corner? A Quick Look at the Literature
No biologic agent is approved to treat COPD, but progress is being made. Key developments of the past few years are summarized in this brief slideshow.
Omalizumab, a monoclonal antibody, became the first biologic therapy for severe asthma when it received FDA approval in 2003. Since then, several other biologic therapies have become available for asthma treatment. No biologic therapy is currently approved for chronic obstructive pulmonary disease (COPD), but progress is being made.
Key developments of the past few years are summarized in the slides below.

Novel therapies addressed underlying pathways. Research into the pathophysiology underlying asthma and COPD has led to novel targeted therapies that address the pathways that cause them, according to a 2018 review. Targets included immunoglobulin E (IgE), interleukin 5 (IL-5), IL-4/IL-13, thymic stromal lymphopoietin, and IL-17. For asthma, the focus was on type 2 inflammation. Novel targeted biologic therapies for COPD had not been approved. New biologics offered an opportunity to provide precision therapies to improve outcomes. Respiratory Care.
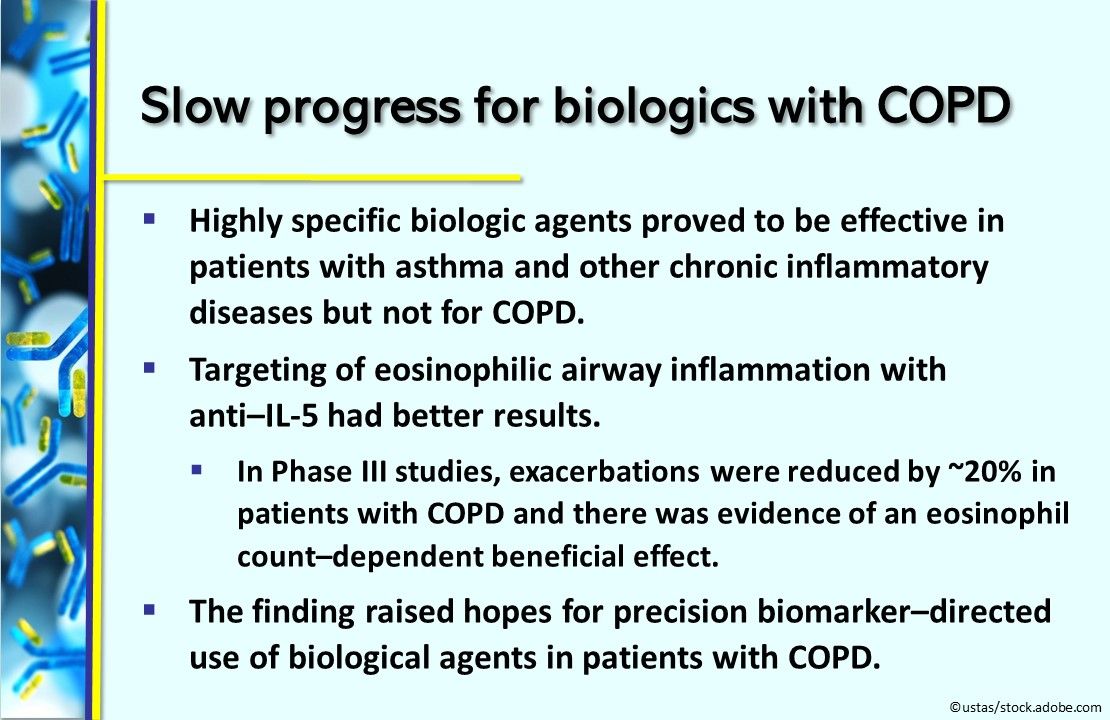
Slow progress for biologics with COPD. Biologic agents that target specific inflammatory pathways, effective in patients with asthma and other chronic inflammatory diseases, showed poor results for COPD. But the targeting of eosinophilic airway inflammation with anti–IL-5 did better. Phase III studies showed a reduction in exacerbations of about 20% in patients with COPD and evidence of a blood eosinophil count–dependent beneficial effect. This finding raised hopes for precision biomarker–directed use of biological agents in patients with COPD. The Journal of Allergy and Clinical Immunology.

Severe obstructive lung disease redefined. In 2019, researchers noted that treatment for severe obstructive lung disease with high-dose inhaled corticosteroids and for severe asthma with oral corticosteroids can have serious adverse effects and that biologic therapies were available only for patients with T2-high phenotypes. They recommended a phenotype- and endotype-focused approach to future research to identify novel biomarkers and potential targets and allow targeted biologic therapies to benefit a greater proportion and range of patients. European Respiratory Journal.

Issues in targeted treatment for severe asthma. The addition of targeted treatment based on phenotyping was a significant change in severe asthma management, said the European Academy of Allergy and Clinical Immunology (EAACI). In 2020 guidelines, the EAACI noted that better understanding of the mechanisms of severe asthma led to a stratified treatment approach, but several issues remain unclear: selection of a biologic, definition of response, strategies to enhance responder rate, treatment duration and regimen, and cost‐effectiveness. European Journal of Allergy and Clinical Immunology.
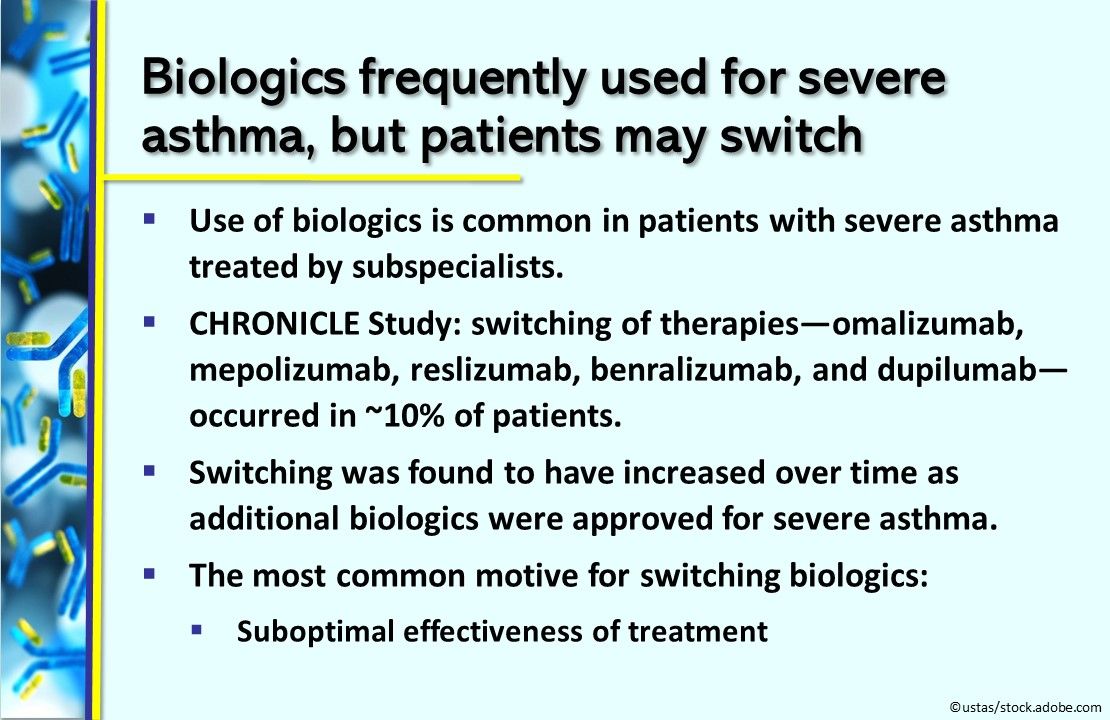
Biologics frequently used for severe asthma, but patients may switch. The use of biologic therapies is common in patients with severe asthma treated by subspecialists. In the CHRONICLE Study, switching of therapies—omalizumab, mepolizumab, reslizumab, benralizumab, and dupilumab—occurred in about 10% of patients. Switching was found to have increased over time with approvals of additional biologics for severe asthma. The most common motive for switching biologics: suboptimal effectiveness of treatment. Chest
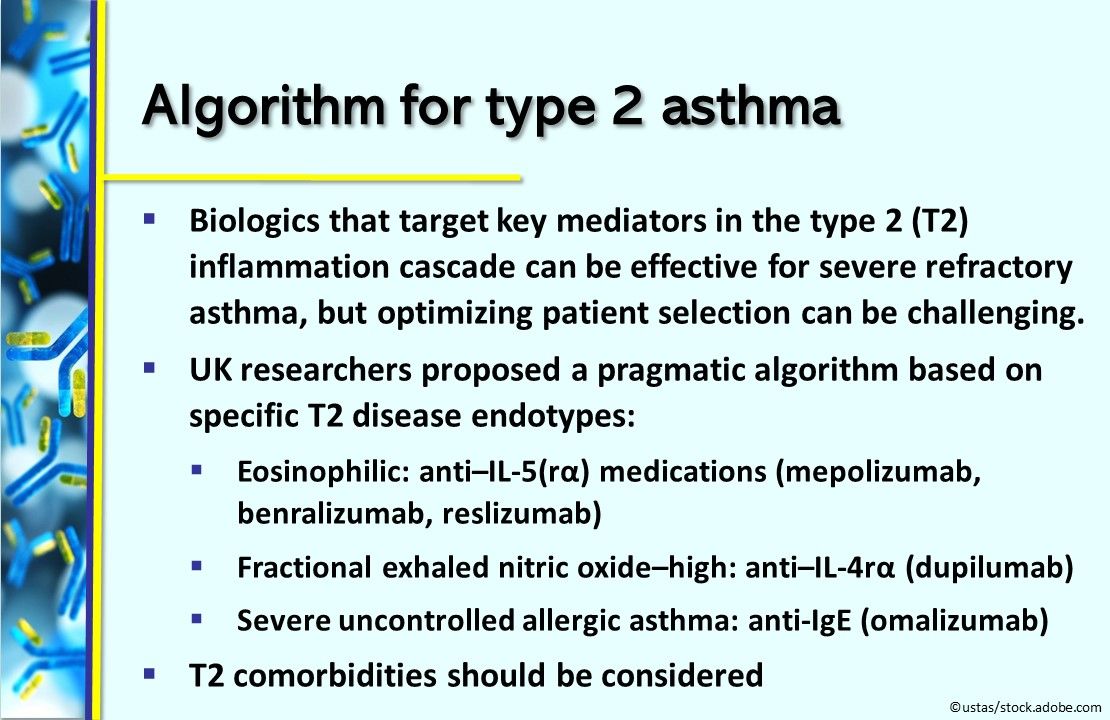
Algorithm for type 2 asthma. Biologics that target key mediators in the type 2 inflammation cascade can be effective for severe refractory asthma, but choosing the optimal one can be challenging. So UK researchers proposed a pragmatic algorithm based on specific type 2 disease endotypes: eosinophilic—anti–IL-5(rα) medications (mepolizumab, benralizumab, reslizumab); fractional exhaled nitric oxide–high—anti–IL-4rα (dupilumab); severe uncontrolled allergic asthma—anti-IgE (omalizumab). Type 2 comorbidities should be considered. The Journal of Allergy and Clinical Immunology: In Practice.

Hyaluronan improves acute COPD exacerbations. In a recent pilot study, inhaled high molecular weight hyaluronan (HMW-HA) benefited patients with severe acute exacerbations of COPD by improving inflammation and lung function and reducing the need for ventilatory support. The findings support the use of a novel class of treatment, matrix biologics, in acute and chronic lung disease. Additional studies are needed to explore the use of HMW-HA in chronic COPD. Respiratory Research.

New biology needed to fuel COPD pipeline. Current COPD treatments are not curative and strategies and drugs in the pipeline still address mostly secondary inflammatory pathways, Johns Hopkins researchers suggested in a recent review. Pipeline strategies and targets include oxidative stress, kinases, phosphodiesterase inhibitors, and interleukins. The authors pointed to a need for a new core biology and ways to incorporate it into therapeutic development. American Journal of Physiology-Lung Cellular and Molecular Physiology.
Related Content:




