© 2025 MJH Life Sciences™ , Patient Care Online – Primary Care News and Clinical Resources. All rights reserved.
ADA 2018: New Basal Insulins go Head-to-Head
How do 2nd generation basal insulin formulations perform vs their predecessors? We report highlights of 3 important studies from the ADA 78th Scientific Sessions.

Studies at the ADA 78th Scientific Sessions included comparisons of first- vs second-generation insulins in the treatment of patients with type 2 diabetes. Summarized in the slides above are 3 late-breaking abstracts: the first head-to-head clinical trial of 2nd generation basal insulins, insulin glargine 300U/mL and insulin degludec, and two trials of their relative effects in real world settings, one involving a switch from first generation insulin products.
- Insulin glargine 300 vs insulin degludec 100 in insulin-naive patients
- Outcomes after switching from first-generation to second-generation insulins
- Real-world comparison of insulin glargine 300 vs insulin degludec 100
Similar glycemic control and less or comparable hypoglycemia with insulin glargine 300 U/ml (GLA-300) vs degludec 100 U/ml (IDeg-100) in insulin-naÑve T2DM on antihyperglycemic drugs ± GLP-1 RAs
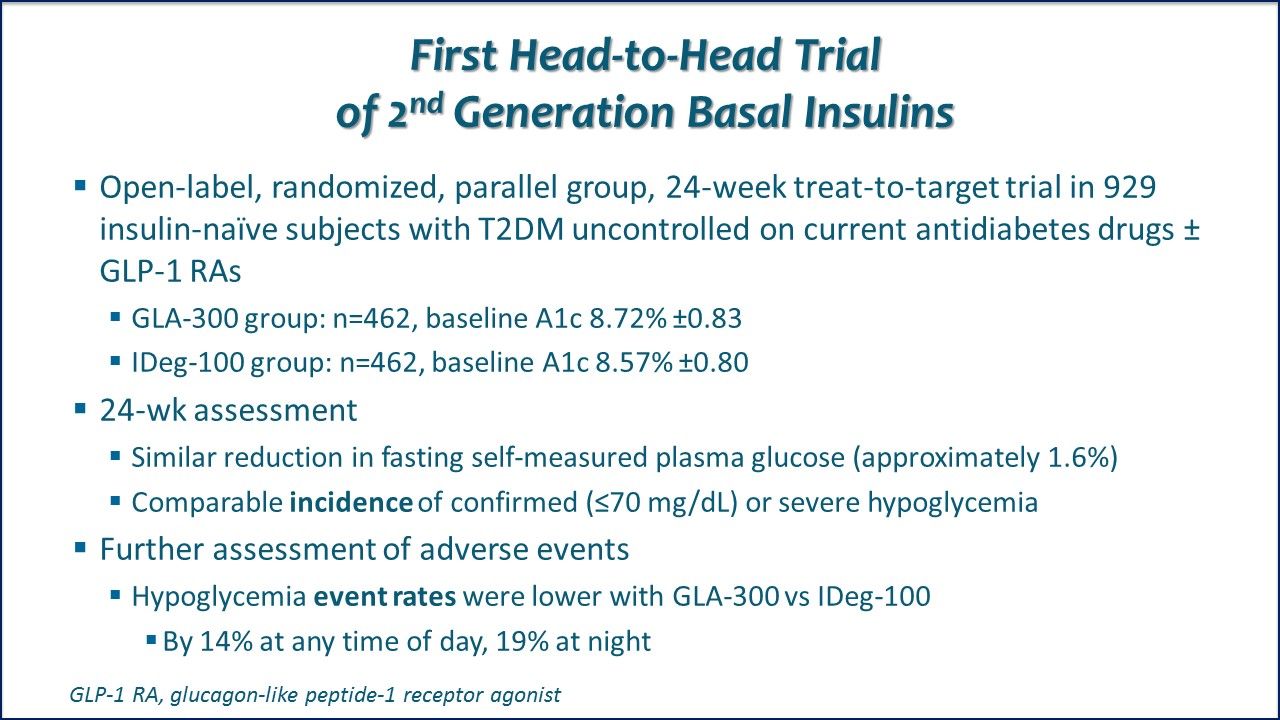
First Head-to-Head Trial of 2nd Generation Basal Insulins. Both 2nd-generation basal insulins (GLA-300 and IDeg-100) provided similar A1c reductions and low risk of hypoglycemia, regardless of concurrently used non-insulin antihyperglycemic drug therapies.

Head-to-Head Trial, Neck-and-Neck Results. Conclusion: Presenting author notes that "GLA-300 provides similar control to IDeg-100, with less or comparable hypoglycemia, in previously inadequately controlled, insulin-naÑve adults with T2DM." Link to ADA abstract.

Real-world clinical outcomes of type 2 diabetes patients switching from insulin glargine 100 U/mL (GLA-100) or insulin detemir (IDet) to insulin glargine 300 U/mL (GLA-300) or insulin degludec (IDeg)
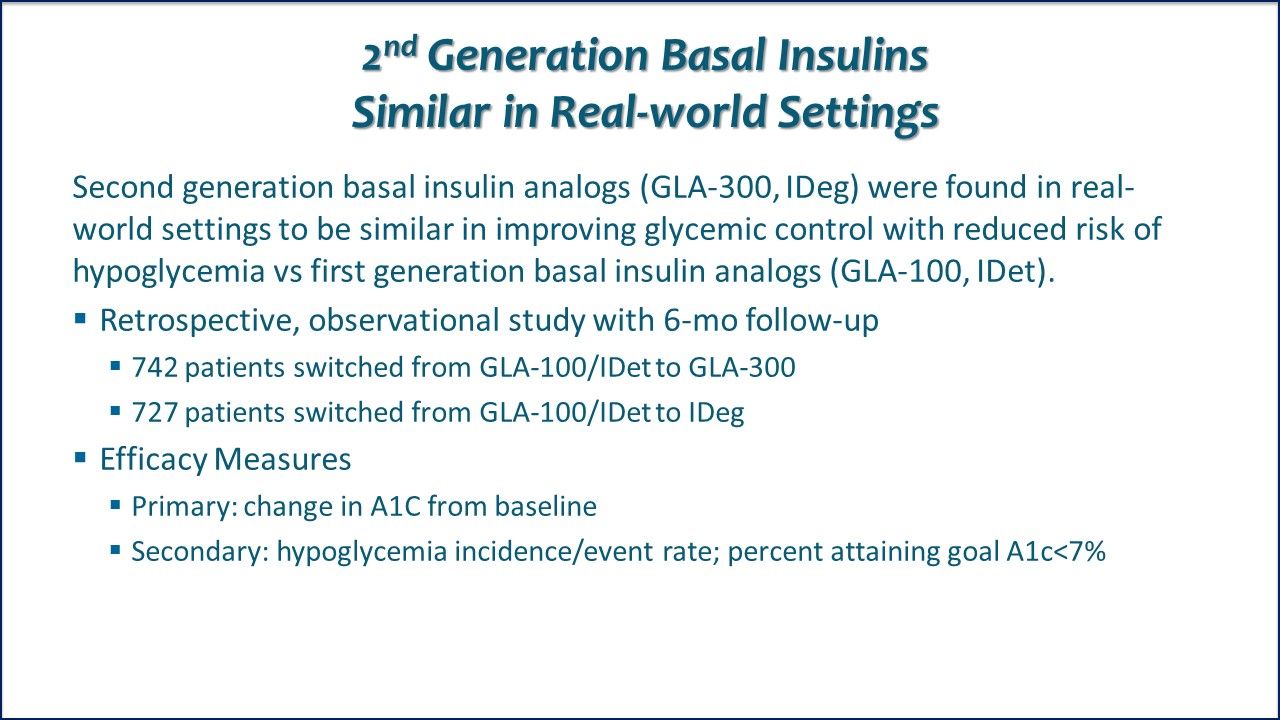
2nd Generation Basal Insulins Similar in Real-world Settings. Second generation basal insulin analogs insulin glargine 300 U/mL (GLA-300) and Insulin Degludec (IDeg) were found in real-world settings to be similar in producing better glycemic control with reduced risk of hypoglycemia compared with first generation basal insulin analogs.
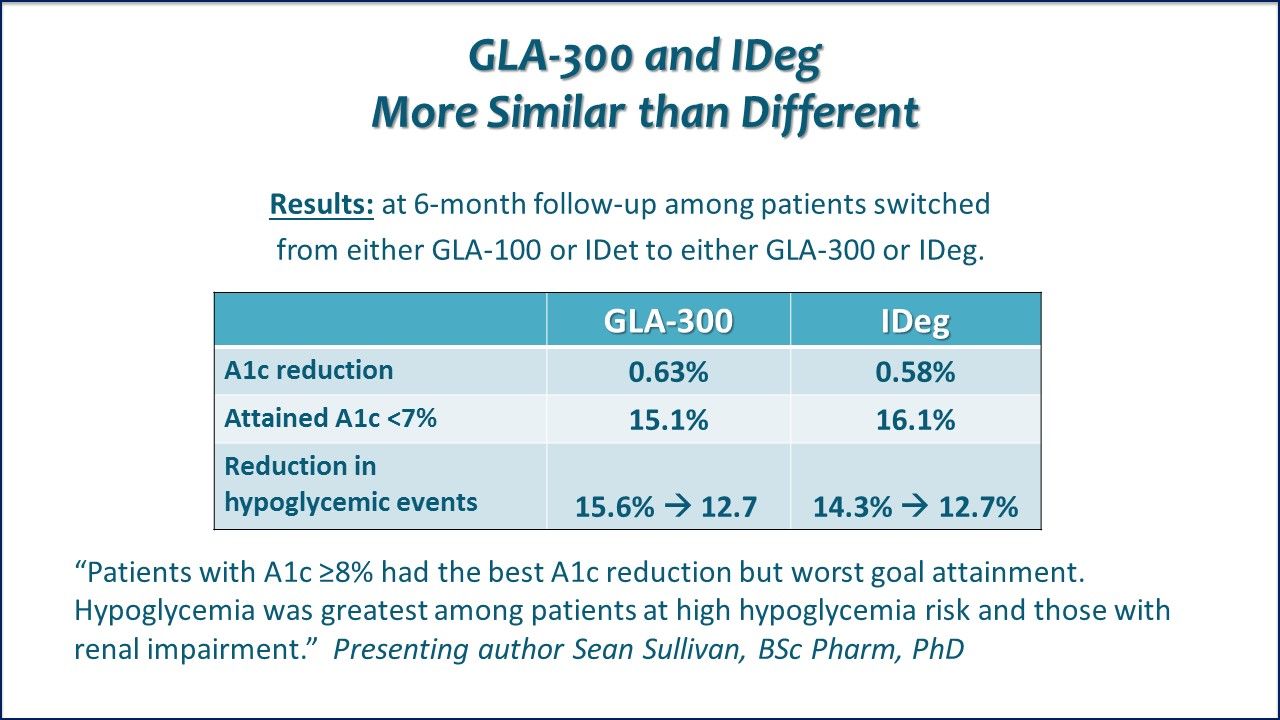
GLA-300 and IDeg, More Similar than Different. Comparable reduction in hypoglycemia incidents when patients switched to GLA-300 or to IDeg:
⪠GLA-300 (across all patients in study): reduced 15.6% to 12.7%
⪠GLA-300 (inpatients/emergency dept): reduced 5.3% to 3.5%
⪠IDeg (across all patients in study): reduced 14.3% to 12.7%
⪠IDeg (inpatients/emergency dept): reduced 4.1% to 3.6%
GLA-300 switchers had lower inpatient/ED event rates: adjusted rate ratio 0.56, p=0.02
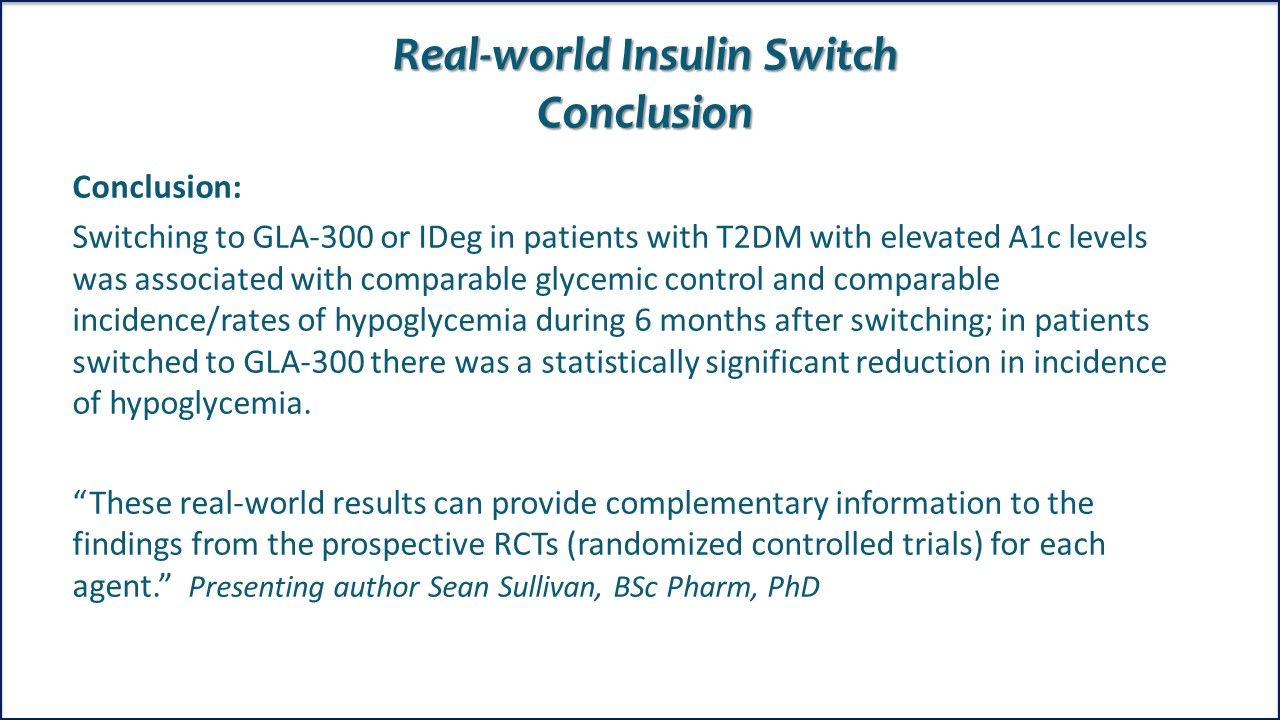
Real-world Insulne Switch Conclusion: Switching to GLA-300 or IDeg in patients with T2DM characterized by elevated A1c levels was associated with comparable glycemic control, comparable incidences and rates of hypoglycemia during 6 months after switching, and significant reduction in hypoglycemia incidence among GLA-300 switchers. Link to ADA Abstract. Link to ADA subgroup analyses abstract.

Clinical outcome assessment of the effectiveness of insulin degludec (Degludec) in real life medical practice (CONFIRM): a comparative effectiveness study of Degludec and insulin glargine 300U/mL (Glargine U300) in insulin-naÑve patients with type 2 diabetes (T2D)

Real-world Clinical Practice, Degludec vs Glargine 300. A retrospective review of electronic health records of adults with T2DM treated with oral antidiabetic drugs (OADs) and either Degludec or GLA-300 found greater glycemic control and lower incidence of hypoglycemia with Degludec.
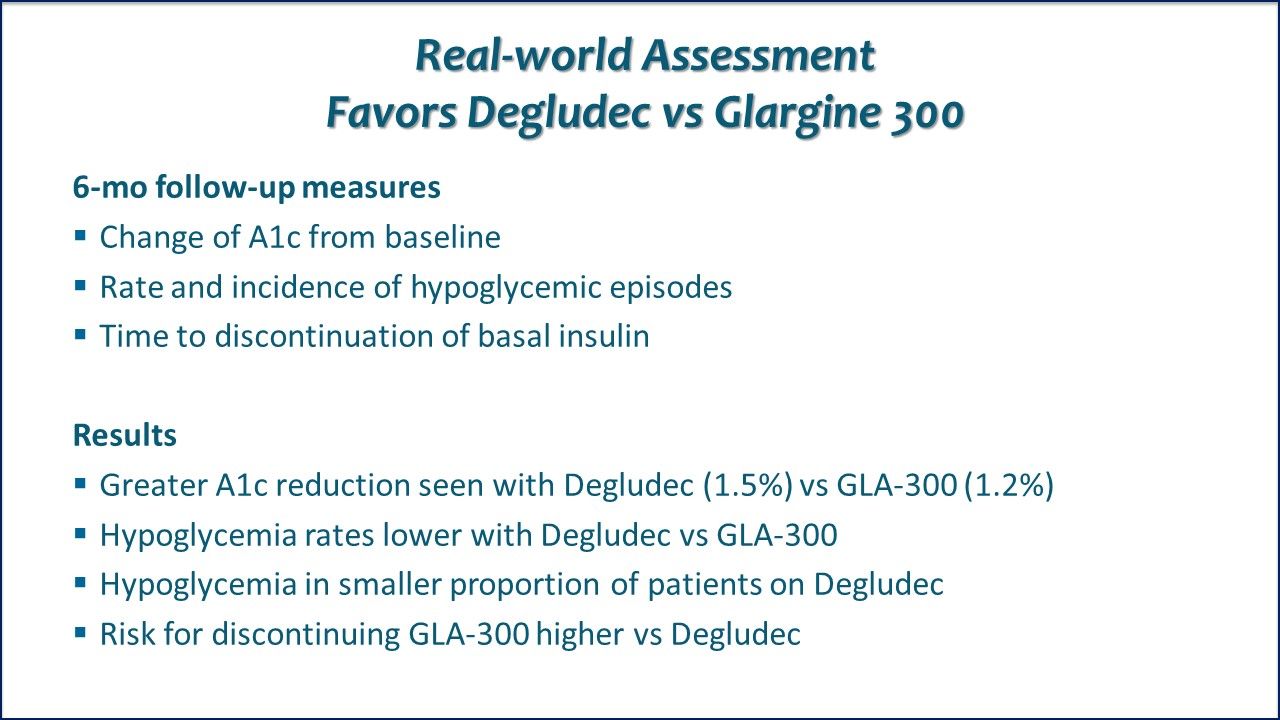
Real-world Assessment Favors Degludec vs Glargine 300. Results of this retrospective electronic health record review differed from the two just reviewed. In adtults with T2DM treated with oral antidiabetic drugs and either Insulin degludec or insulin glargine 300, Degludec was associated with greater glycemic control and lower incidence of hypoglycemia than insulin glargine.
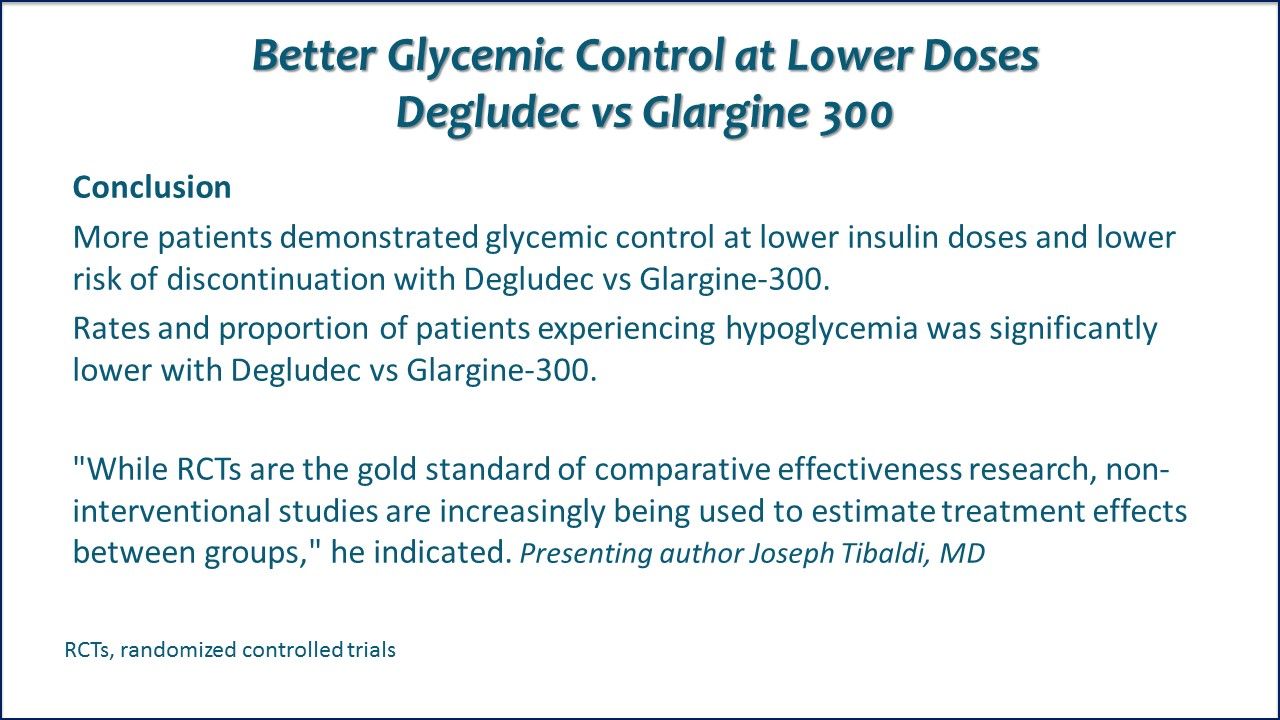
Better Glycemic Control at Lower Doses with Degludec vs Glargine 300. Conclusion. More patients demonstrated glycemic control at lower insulin doses and lower risk of discontinuation with degludec vs glargine-300. Rates and proportion of patients experiencing hypoglycemia was significantly lower with degludec vs glargine-300. Link to ADA Abstract.



