© 2025 MJH Life Sciences™ , Patient Care Online – Primary Care News and Clinical Resources. All rights reserved.
8 Questions on Diabetes Drugs for Cardioprotection
Slideshow
Cardioprotective effects of sodium–glucose cotransporter 2 inhibitors (SGLT2is) and glucagon-like peptide 1 receptor agonists (GLP-1 RAs) in patients with type 2 diabetes (T2D) and atherosclerotic cardiovascular disease (ASCVD) have been reported in a number of high-profile, landmark clinical trials.
Take this quick quiz to test your knowledge of key findings.

1. For patients with T2D who have established ASCVD or CKD, the ADA “Standards of Medical Care in Diabetes—2021” recommends an SGLT2i or GLP-1 RA to do what?

Answer: C. Both. The ADA standard recommends an SGLT2i or GLP-1 RA with demonstrated CVD benefit as part of the comprehensive CV risk reduction or glucose-lowering regimen, or both, for patients with T2D who have established ASCVD or established CKD.

Answer: E. All of the above. Numerous trials have reported statistically significant reductions in CV events for these FDA-approved GLP-1 RAs and for the FDA-approved SGLT2is empagliflozin, canagliflozin, and dapagliflozin.

3. Results of the EMPEROR-Preserved phase 3 trial established which SGLT2i as the first and only therapy to significantly reduce the risk of the composite of CV death or hospitalization for heart failure in adults who have heart failure with reduced ejection fraction?

Answer: B. Empagliflozin. When added to earlier results, the findings demonstrate efficacy in all forms of heart failure regardless of ejection fraction
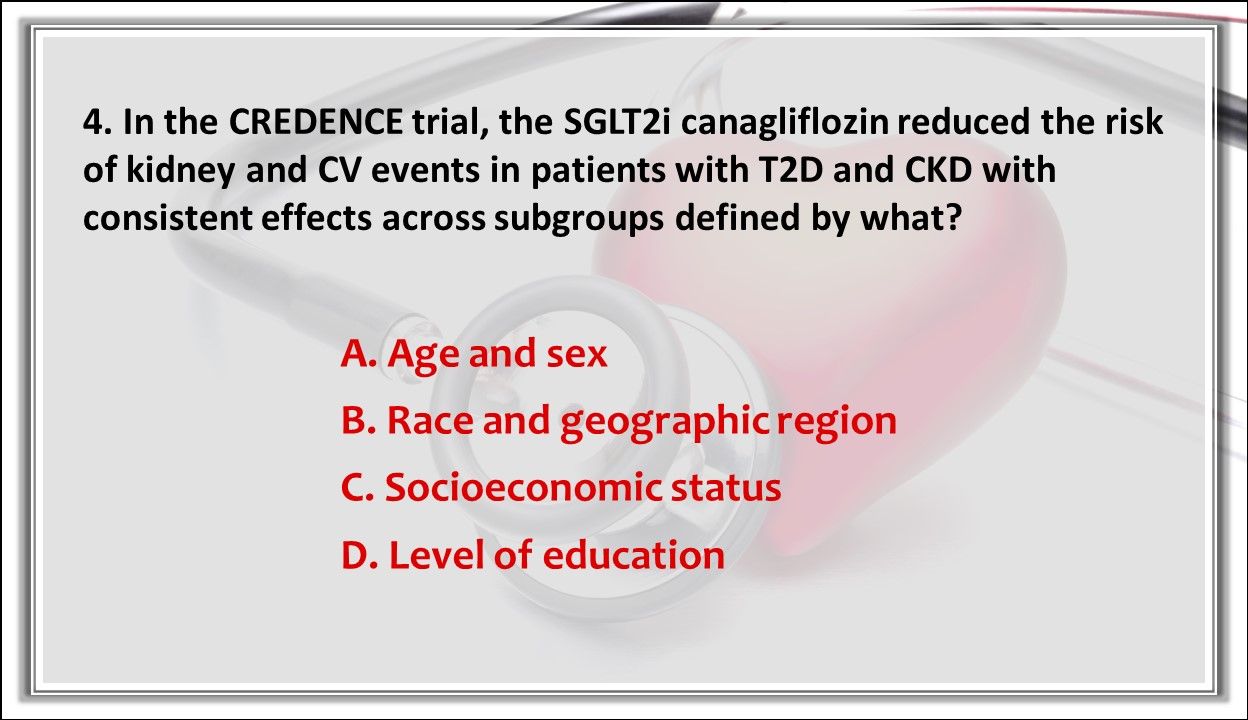
4. In the CREDENCE trial, the SGLT2i canagliflozin reduced the risk of kidney and CV events in patients with T2D and CKD with consistent effects across subgroups defined by what?

Answer: A. Age and sex. Also, there were no differences in safety outcomes across subgroups defined by age and sex.
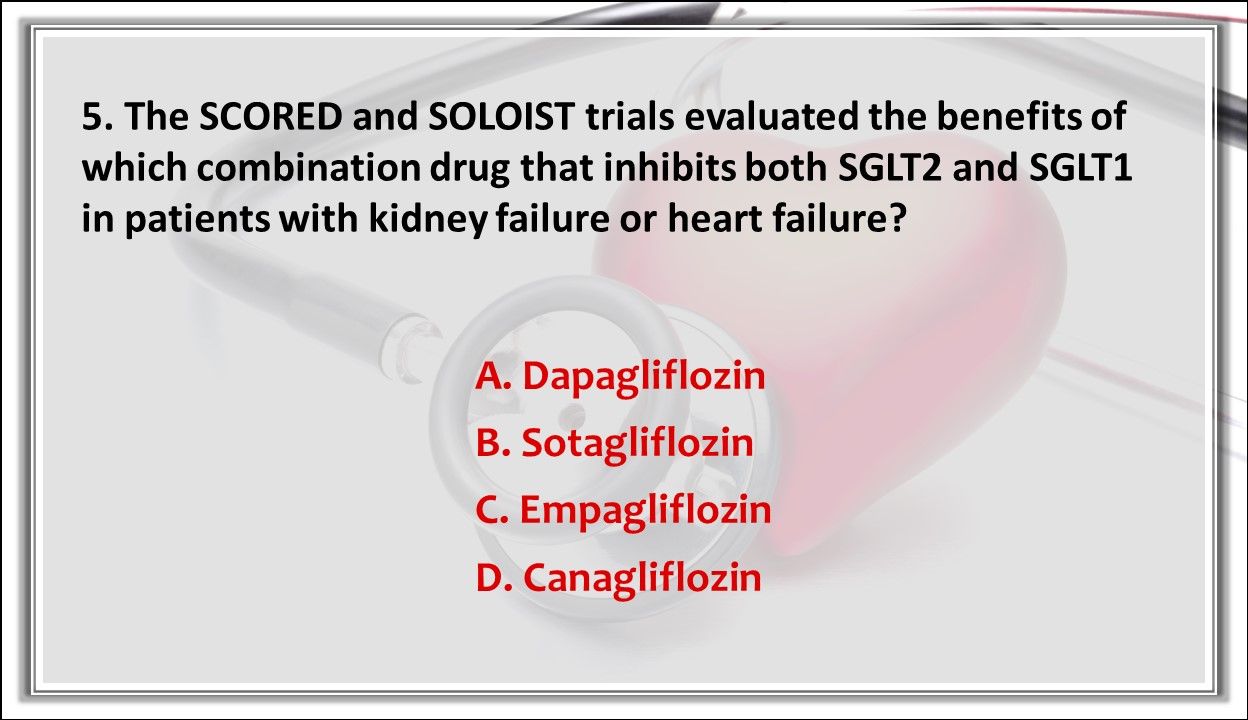
5. The SCORED and SOLOIST trials evaluated the benefits of which combination drug that inhibits both SGLT2 and SGLT1 in patients with kidney failure or heart failure?

Answer: B. Sotagliflozin. Sotagliflozin showed significant benefits in reducing heart failure, heart attack, and stroke in patients with T2D with CKD or heart failure.
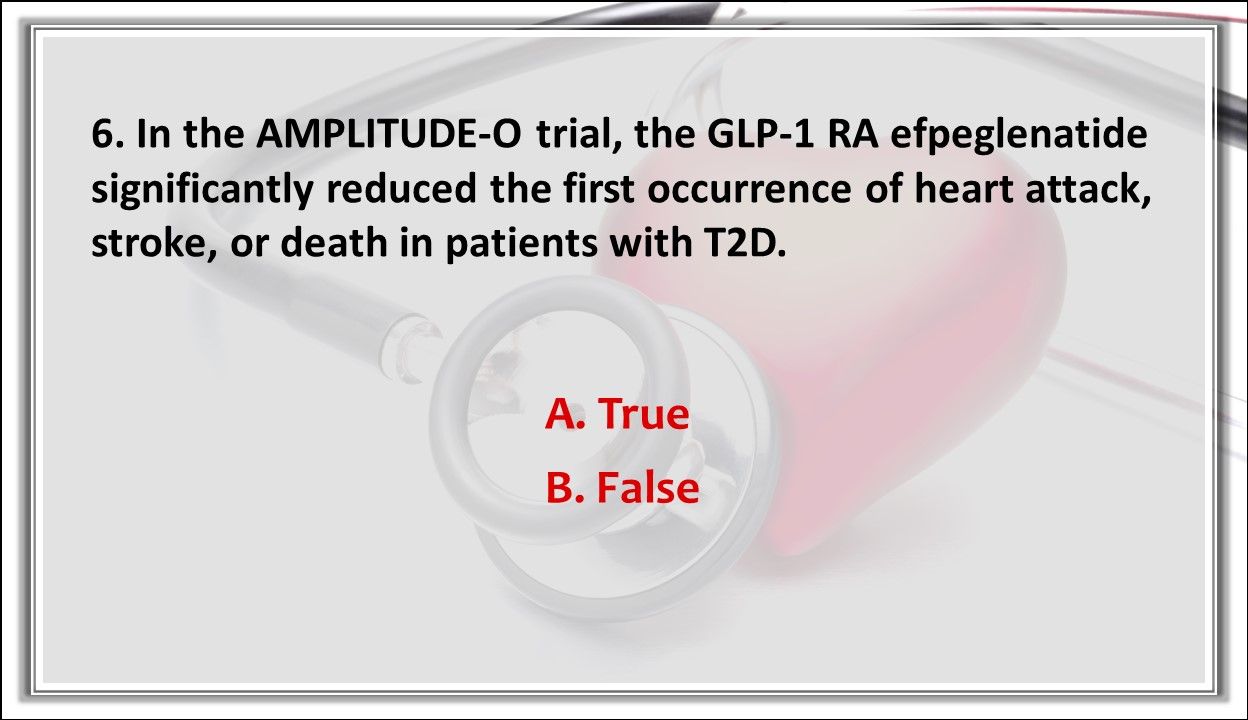
6. TRUE OR FALSE? In the AMPLITUDE-O trial, the GLP-1 RA efpeglenatide significantly reduced the first occurrence of heart attack, stroke, or death in patients with T2D.

Answer: A. True. Weekly subcutaneous injections of efpeglenatide at a dose of 4mg or 6 mg significantly reduced the first occurrence of heart attack, stroke, or death and reduced progression of kidney disease in patients who had a history of CVD or current kidney disease plus at least 1 other CV risk factor.
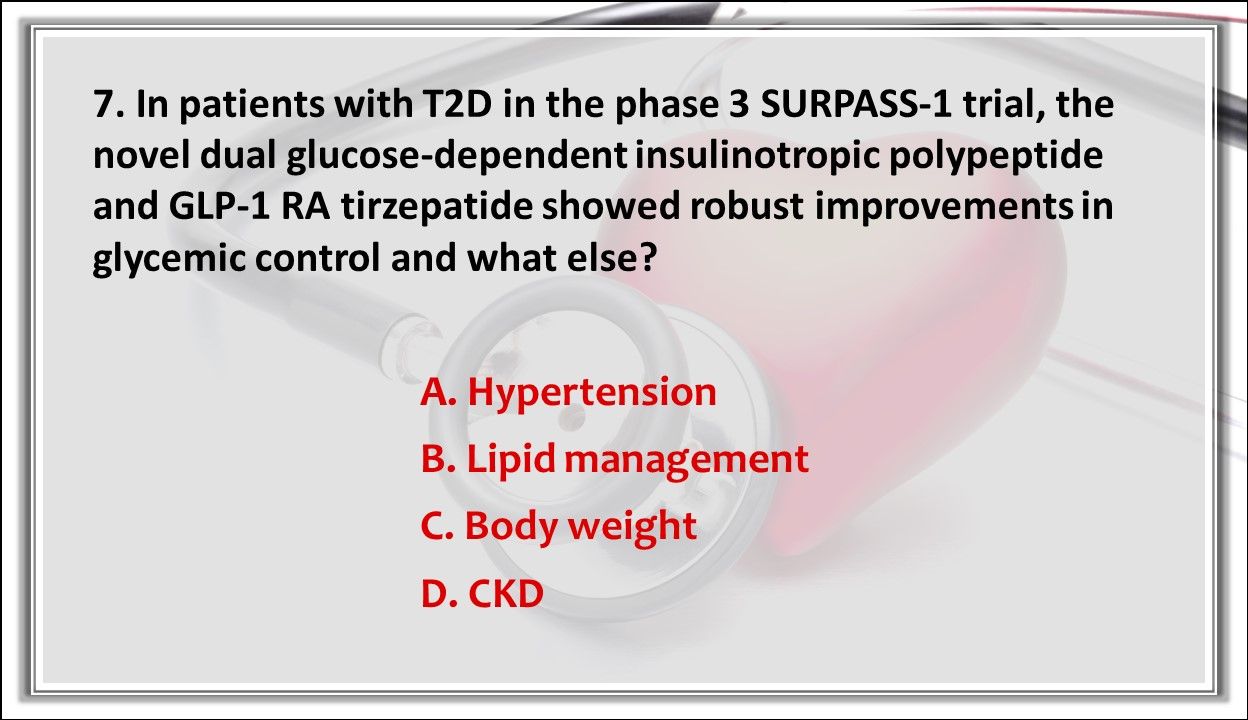
7. In patients with T2D in the phase 3 SURPASS-1 trial, the novel dual glucose-dependent insulinotropic polypeptide and GLP-1 RA tirzepatide showed robust improvements in glycemic control and what else?

Answer: C. Body weight. Tirzepatide showed improvements in body weight without increased risk of hypoglycemia. The safety profile was consistent with GLP-1 RA, indicating a potential monotherapy use for T2D treatment.
8. The DARE-19 trial explored the use of the SGLT2i dapagliflozin for patients who have hypertension, CVD, heart failure, T2D, or CKD and are hospitalized with what condition?

Answer: D. COVID-19. Numerically fewer patients treated with dapagliflozin experienced organ failure or death, but treatment did not achieve statistically significant reduction in organ failure or death or significantly improve clinical recovery.
For more information on these 8 questions and answers, check out Diabetes Drugs and Cardioprotection: A Summary for Primary Care.
Related Content:




