© 2025 MJH Life Sciences™ , Patient Care Online – Primary Care News and Clinical Resources. All rights reserved.
11 New FDA-Approved Primary Care Drugs
In the past few months, the FDA has approved a number of drugs for a variety of primary care disorders. Here’s a look at 11 of the newest.

Since September 2014, the FDA has approved nearly a dozen medications indicated for primary care diseases. A brief look at 11 of the newest approvals, starting with the most recent.
Pazeo (olopatadine hydrochloride) is a mast cell stabilizer for treatment of ocular itching associated with allergic conjunctivitis. Pazeo solution is dosed 1 drop daily, and was approved with efficacy data at 24 hours, post dose.Approved 1/30/15; Manufacturer, Alcon Laboratories, Inc.

Prestalia (amlodipine besylate/perindopril arginine) combines a calcium channel blocker and long-acting ACE inhibitor for treatment of hypertension. The first fixed-dose combination of these two medications, this medication may be used in patients whose blood pressure is not adequately controlled with monotherapy. May may be used as initial therapy if a patient is likely to need multiple drugs to achieve blood pressure goals.Approved 1/21/15; Manufacturer, Symplmed

Savaysa (edoxaban) is an oral, once-daily factor Xa inhibitor anticoagulant indicated for prevention of stroke and systemic embolism in patients with nonvalvular atrial fibrillation, and for the treatment of deep vein thrombosis and pulmonary embolism.Approved: 1/8/15; Manufacturer: Daiichi Sankyo Company

Saxenda (liraglutide) is a once-daily glucagon-like peptide-1 (GLP-1) analogue for treatment of obesity. The drug is approved for use in adults with a BMI of 30 or greater or adults with a BMI of 27 or greater (overweight) who have at least one weight-related condition such as hypertension, type 2 diabetes, or dyslipidemia.Approved: 12/23/14; Manufacturer: Novo Nordisk Inc.
Rapivab (peramivir) is an influenza virus neuraminidase inhibitor indicated for treatment of acute uncomplicated influenza in adults. Administered as a single IV dose. For patients 18 years and older who have acute uncomplicated influenza and who have shown symptoms of flu for no more than 2 days.Approved 12/19/14; Manufacturer, BioCryst Pharmaceuticals, Inc.
Gardasil 9 (human papillomavirus 9-valent vaccine, recombinant) is a 9-valent HPV vaccine for the prevention of cervical, vulvar, vaginal, and anal cancers caused by HPV types 6, 11, 16, 18, 31, 33, 45, 52, and 58. Covering 9 HPV types, 5 more HPV types than Gardasil (previously approved by the FDA), Gardasil 9 has the potential to prevent approximately 90% of cervical, vulvar, vaginal, and anal cancers.Approved 12/10/14; Manufacturer, MercK & Co, Inc.
Zerbaxa (ceftolozane and tazobactam) is a cephalosporin and beta-lactamase inhibitor combination for treatment of complicated intra-abdominal infections and complicated urinary tract infections.Approved 12/19/14; Manufacturer; Cubist Pharmaceuticals, Inc.
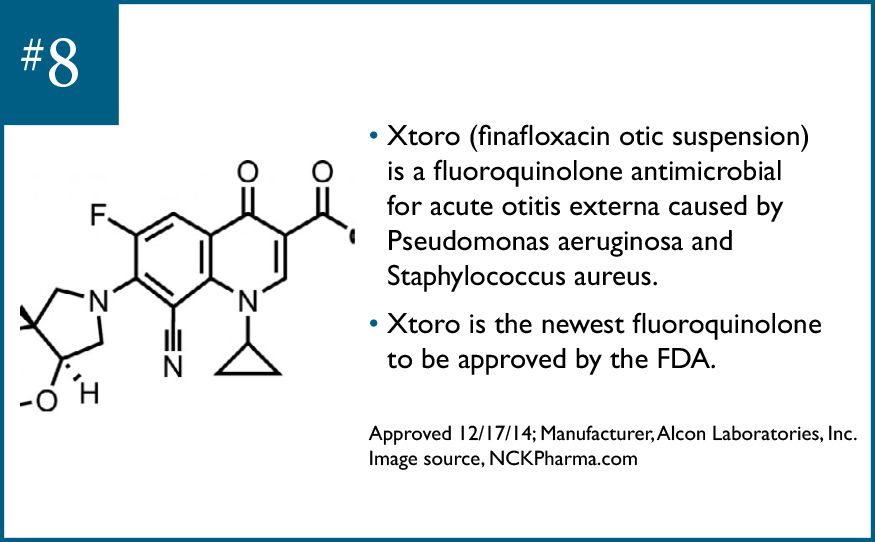
Xtoro (finafloxacin otic suspension) is a fluoroquinolone antimicrobial for the treatment of acute otitis externa caused by P aeruginosa and Staph aureus. Xtoro is the newest fluoroquinolone to be approved by the FDA.Approved 12/17/14; Manufacturer, Alcon Laboratories, Inc.

Onexton (benzoyl peroxide/clindamycin phosphate) is an antimicrobial and lincosamide antibacterial combination for once-daily topical treatment of acne vulgaris.Approved: 11/25/14; Manufacturer, Valeant Pharmaceuticals International, Inc.

Trumenba (meningococcal group B vaccine) is indicated for active immunization to prevent invasive disease caused by N meningitidis serogroup B in individuals 10 -25 years of age.Approved 10/19/14; Manufacturer, Pfizer Inc.
Contrave (bupropion/naltrexone) is an aminoketone antidepressant and opioid antagonist combination indicated as an adjunct to a reduced-calorie diet and increased physical activity for chronic weight management. Approved for adults with a BMI of 30 or greater (obesity) or adults with a BMI of 27 or greater (overweight) who have at least one weight-related condition, such as hypertension, type 2 diabetes, or dyslipidemia.Approved 9/10/14; Manufaturer, Orexigen Therapeutics, Inc.
In the past 6 months, the FDA approved a number of medications for a variety of primary care disorders. Here’s a brief look at 11 of the most recent (in order of the date they were approved).
