© 2025 MJH Life Sciences™ , Patient Care Online – Primary Care News and Clinical Resources. All rights reserved.
10 New Drugs for Primary Care: Q4 2019
Two first-in-class migraine drugs, the first generic version of NuvaRing®, and 7 more fourth quarter FDA-approved drugs for primary care.
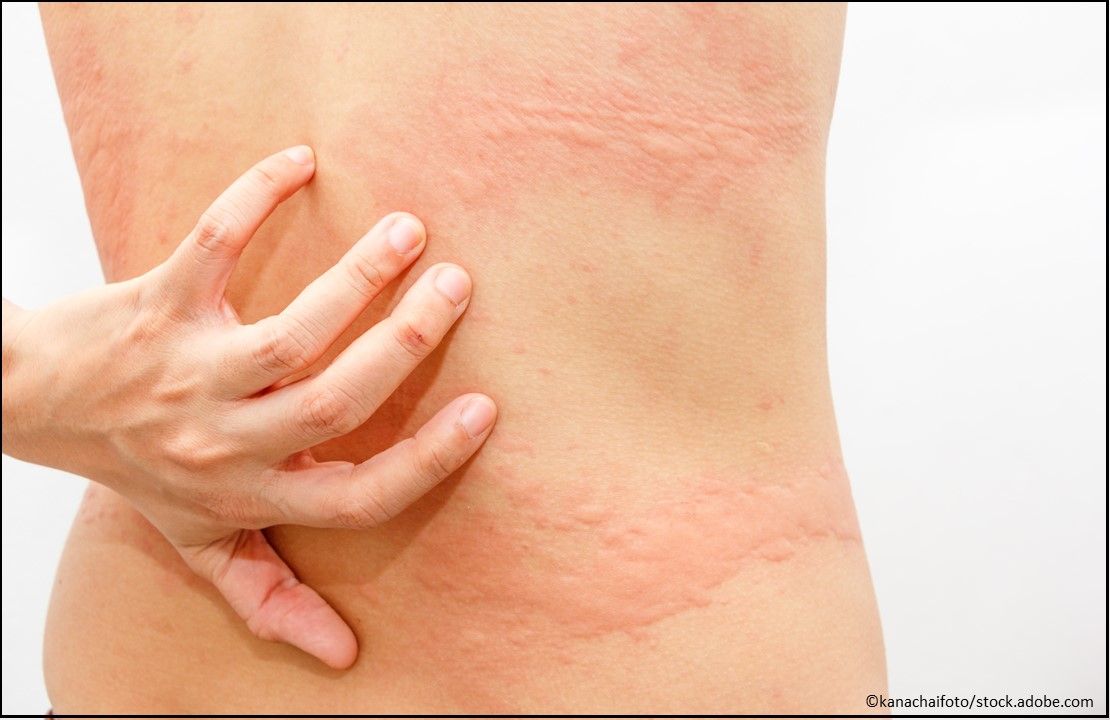
Urticaria: Quzyttir (cetirizine hydrochloride) Injection, 10 mg/mL, is a histamine-1 receptor antagonist indicated for the treatment of acute urticaria in adults and children aged ≥6 months. (Approved 10/4/19, TerSera Therapeutics LLC)
For full prescribing information, please click here.
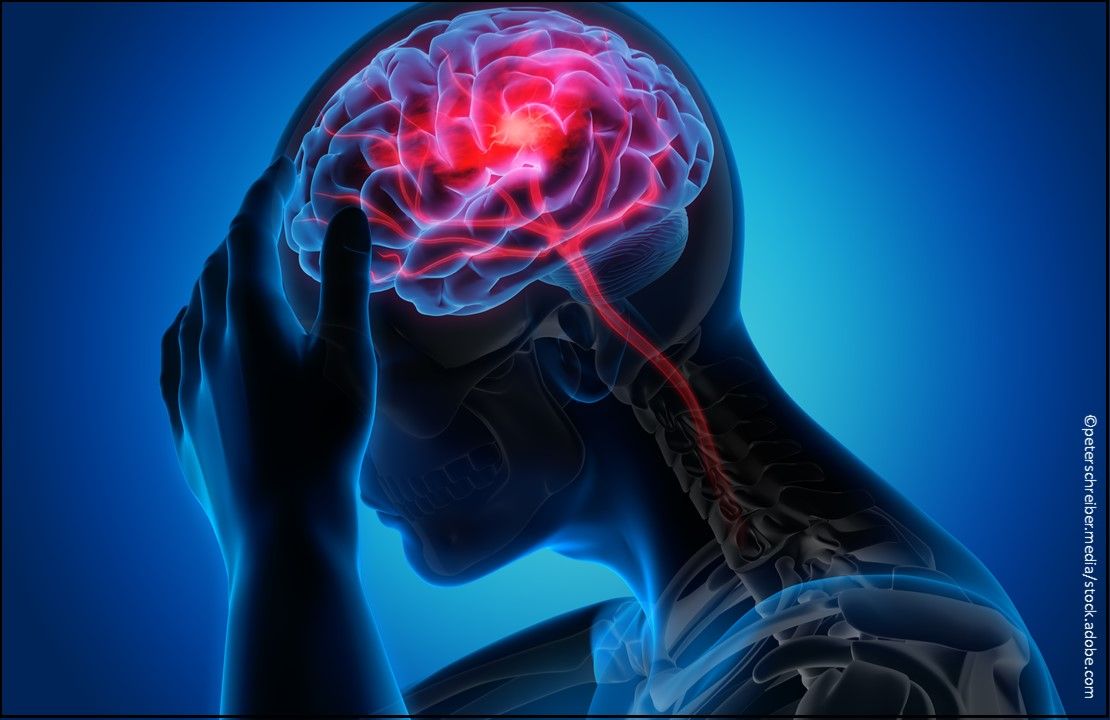
Migraine: Reyvow (lasmiditan) Tablets, a first-in-class serotonin (5-HT)1F receptor agonist indicated for the acute treatment of migraine with or without aura in adults; available in 50 mg and 100 mg tablets and can be prescribed in oral doses of 50 mg, 100 mg, and 200 mg as needed. (Approved 10/11/19, Eli Lilly and Company)
For full prescribing information, please click here.

Pregnancy Prevention: EluRyng (ethinyl estradiol, 2.7 mg and etonogestrel, 11.7 mg) Vaginal Ring, the first generic version of NuvaRing® to receive FDA approval, is an estrogen/progestin combination hormonal contraceptive to prevent pregnancy. (Approved 10/11/19, Amneal Pharmaceuticals, Inc.)
For full prescribing information, please click here.
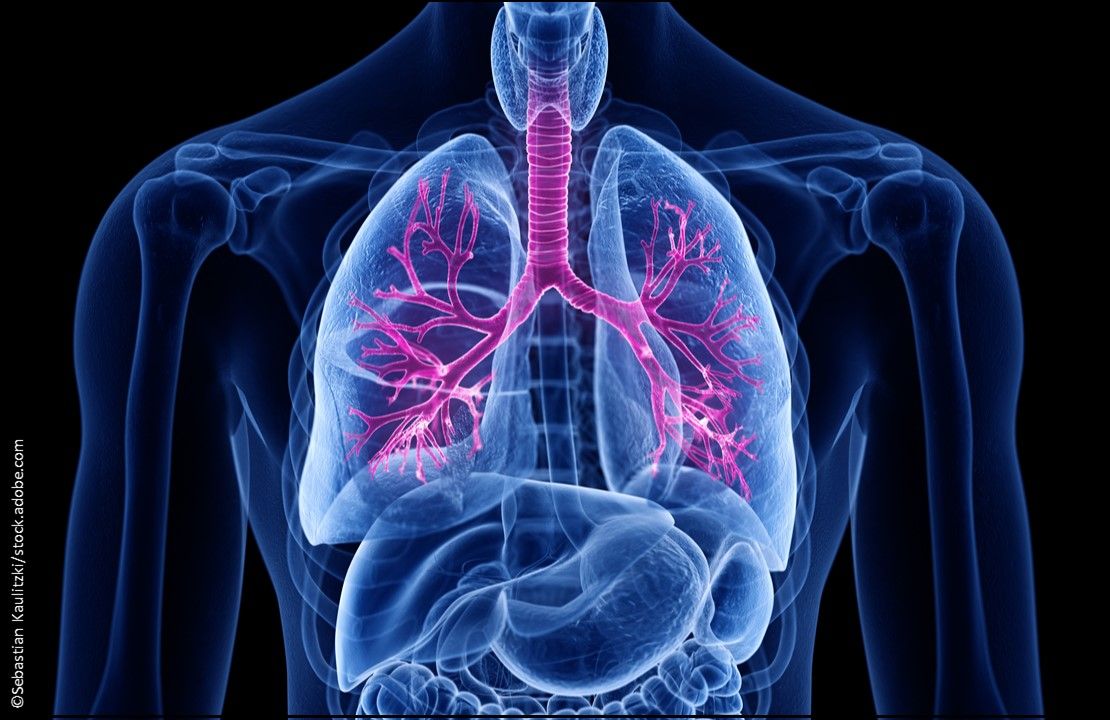
Cystic Fibrosis (CF): Trikafta (elexacaftor 100 mg, tezacaftor 50 mg, ivacaftor 75 mg and ivacaftor, 75 mg) Tablets, the first triple combination therapy approved to treat patients aged ≥12 years who have at least 1 F508del mutation in the CF transmembrane conductance regulator gene, the most common CF mutation. (Approved 10/21/19, Vertex Pharmaceuticals)
For full prescribing information, please click here.
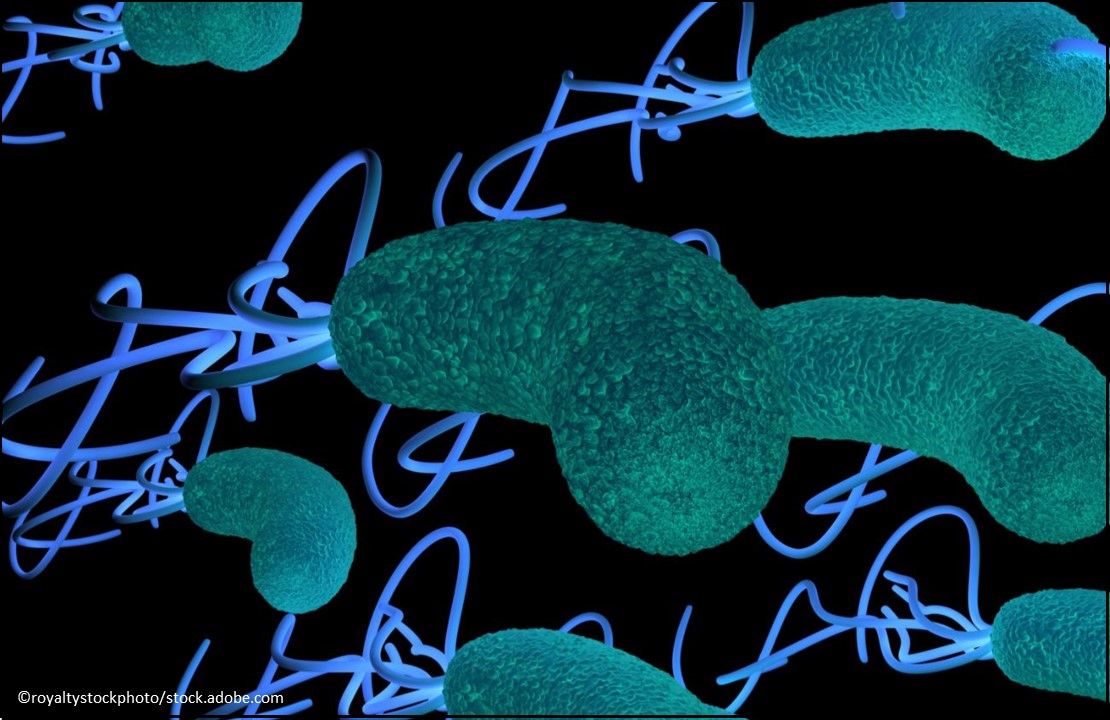
Helicobacter pylori Infection: Talicia (omeprazole magnesium 10 mg, amoxicillin 250 mg, and rifabutin 12.5 mg) Capsules, a delayed-release, fixed-drug combination for the treatment of H. pylori infection in adults that is administered in 4-capsule doses every 8 hours with food for 14 days. (Approved 11/4/19, RedHill Biopharma Ltd.)
For full prescribing information, please click here.
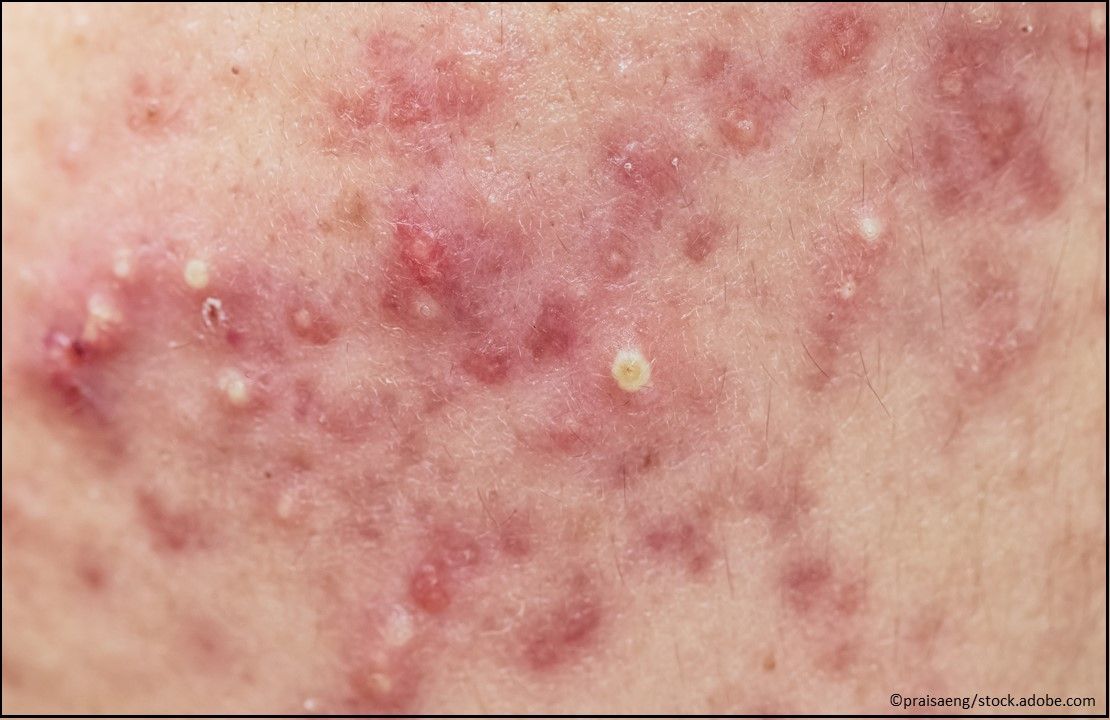
Acne: Amzeeq (minocycline) Topical Foam (formerly FMX101), 4%, a novel tetracycline-class drug indicated for the treatment of inflammatory lesions of non-nodular moderate-to-severe acne vulgaris in patients aged ≥9 years. It is the first topical minocycline to be approved for any condition. (Approved 11/18/19, Foamix Pharmaceuticals)
For full prescribing information, please click here.
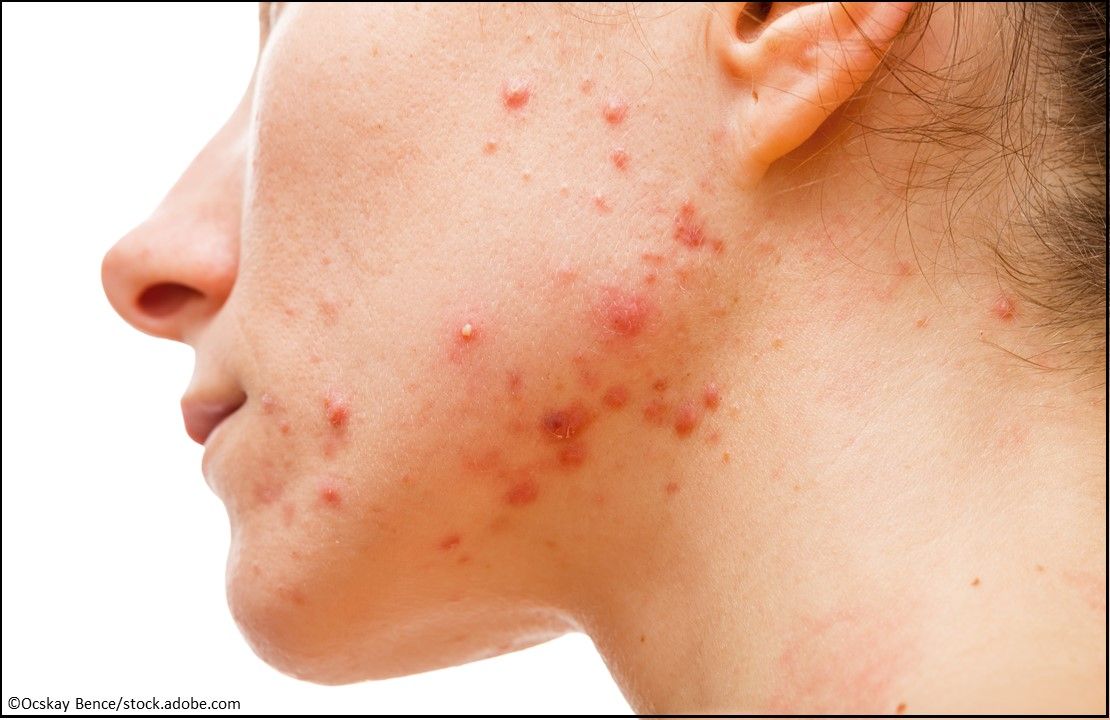
Acne: Arazlo (tazarotene) Lotion, 0.045%, the first tazarotene topical treatment available in a lotion form to treat acne vulgaris in patients aged ≥9 years. (Approved 12/18/19, Bausch Health Companies Inc.)
For full prescribing information, please click here.
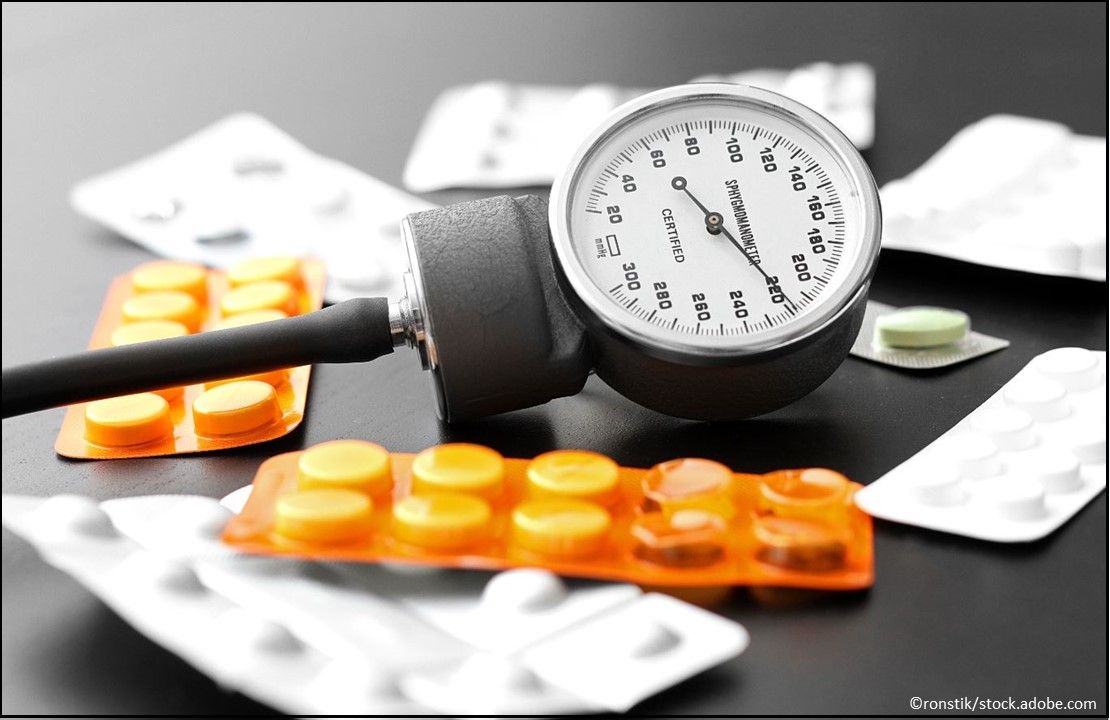
Hypertension: Conjupri (levamlodipine maleate) Tablets, 1.25 mg, 2.5 mg, 5 mg, a calcium channel blocker that may be used alone or combined with other antihypertensive medication for the treatment of hypertension; adult recommended starting dose is 2.5 mg once daily with maximum dose 5 mg once daily and pediatric starting dose is 1.25 mg-2.5 mg once daily. (Approved 12/19/19, CSPC Pharmaceutical Group Limited)
For full prescribing information, please click here.

Insomnia: Dayvigo (lemborexant) Tablets, 5 mg, 10 mg, a dual orexin receptor antagonist to treat adults with insomnia (characterized by difficulties with sleep onset and/or sleep maintenance) to be taken no more than once/night immediately before bedtime. (Approved 12/20/19, Eisai Co., Ltd.)
For full prescribing information, please click here.

Migraine: Ubrelvy (ubrogepant) Tablets, 50 mg, 100 mg, the first and only FDA-approved oral calcitonin gene-related peptide receptor antagonist for the acute treatment of migraine with or without aura in adults. (Approved 12/23/19, Allergan plc)
For full prescribing information, please click here.
For a quick review of primary care drugs approved in the first quarter of 2019, check out 10 New Drugs for Primary Care: Q1 2019
For a quick review of primary care drugs approved in the second quarter of 2019, check out 7 New Drugs for Primary Care: Q2 2019
For a quick review of primary care drugs approved in the third quarter of 2019, check out 10 New Drugs for Primary Care: Q3 2019
While 2019 may be over, numerous medications were approved by the US Food and Drug Administration (FDA) during the fourth quarter of 2019 for conditions often seen in primary care.Below, you will find details on the first and only FDA-approved oral calcitonin gene-related peptide receptor antagonist for the treatment of migraine; the first topical minocycline to be approved for any condition; the first generic version of NuvaRing®; and 7 more.Â
Related Content:




